Abstract
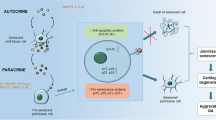
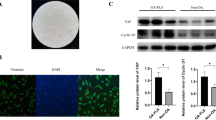
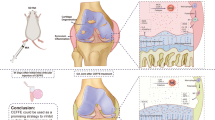
Osteoarthritis (OA) mainly occurs in the elderly population and seriously affects their quality of life (QOL). Strontium (Sr) ions have shown positive effects on bone tissue and are promising for OA treatment. However, the adequate treatment dosage and underlying mechanisms are unclear. This study investigated the effects and underlying mechanisms of different concentrations of Sr ions in a mouse model of OA induced by destabilization of the medial meniscus (DMM) surgery. DMM-induced OA mice received intra-articular injections of different concentrations of Sr ions, and a suitable concentration of Sr ions was found to improve OA. Furthermore, we investigated the mechanism by which Sr ions mediate senescence and autophagy in fibroblast-like synoviocytes (FLSs) in the synovial tissues of DMM-induced OA mice. OA mice treated with 10 µl of 5 mmol/L SrCl2 showed the greatest improvement in pain-related behavior and cartilage damage. In addition, in vivo and in vitro experiments revealed that Sr ions inhibit senescence and improve the autophagic function of FLSs. We also found that enhancement of the autophagic function of FLSs could effectively slow down senescence. Therefore, we show that Sr ions through the AMPK/mTOR/LC3B-II signal axis improve FLSs autophagy function and delay FLSs senescence, and furthermore, improve OA. These results suggest that senescence and autophagy function of FLSs may serve as promising targets for OA treatment, and that Sr ions may inhibit OA progression through these two targets.





Similar content being viewed by others
References
Abramoff B, Caldera FE (2020) Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am 104(2):293–311. https://doi.org/10.1016/j.mcna.2019.10.007
Sacitharan PK (2019) Ageing and Osteoarthritis. Subcell Biochem 91:123–159. https://doi.org/10.1007/978-981-13-3681-2_6
Jones IA, Togashi R, Wilson ML et al (2019) Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol 15(2):77–90. https://doi.org/10.1038/s41584-018-0123-4
Liang Y, Xu X, Xu L et al (2021) Non-surgical osteoarthritis therapy, intra-articular drug delivery towards clinical applications. J Drug Target 29(6):609–616. https://doi.org/10.1080/1061186x.2020.1870231
Chen G, Xu B, Xie J et al (2019) Comparison of Clinical and Biomechanical Outcomes between Partial Fibulectomy and Drug Conservative Treatment for Medial Knee Osteoarthritis. Biomed Res Int 2019:4575424. https://doi.org/10.1155/2019/4575424
Hunter DJ, Bierma-Zeinstra S (2019) Osteoarthritis. Lancet 393(10182):1745–1759. https://doi.org/10.1016/s0140-6736(19)30417-9
Huang H, Lou Z, Zheng S et al (2022) Intra-articular drug delivery systems for osteoarthritis therapy: shifting from sustained release to enhancing penetration into cartilage. Drug Deliv 29(1):767–791. https://doi.org/10.1080/10717544.2022.2048130
Felson DT, McLaughlin S, Goggins J et al (2003) Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 139(5 Pt 1):330–336. https://doi.org/10.7326/0003-4819-139-5_part_1-200309020-00008
Sharma AR, Jagga S, Chakraborty C et al (2020) Fibroblast-Like-Synoviocytes Mediate Secretion of Pro-Inflammatory Cytokines via ERK and JNK MAPKs in Ti-Particle-Induced Osteolysis. Mater (Basel) 13(16). https://doi.org/10.3390/ma13163628
Park H, Lee HR, Shin HJ et al (2022) p16INK4a-siRNA nanoparticles attenuate cartilage degeneration in osteoarthritis by inhibiting inflammation in fibroblast-like synoviocytes. Biomater Sci 10(12):3223–3235. https://doi.org/10.1039/d1bm01941d
Chen X, Gong W, Shao X et al (2022) METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann Rheum Dis 81(1):87–99. https://doi.org/10.1136/annrheumdis-2021-221091
Malaise O, Paulissen G, Deroyer C et al (2021) Influence of Glucocorticoids on Cellular Senescence Hallmarks in Osteoarthritic Fibroblast-like Synoviocytes. J Clin Med 10(22). https://doi.org/10.3390/jcm10225331
Greene MA, Loeser RF (2015) Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 23(11):1966–1971. https://doi.org/10.1016/j.joca.2015.01.008
Wong SQ, Kumar AV, Mills J et al (2020) Autophagy in aging and longevity. Hum Genet 139(3):277–290. https://doi.org/10.1007/s00439-019-02031-7
Mizushima N, Levine B (2020) Autophagy in Human Diseases. N Engl J Med 383(16):1564–1576. https://doi.org/10.1056/NEJMra2022774
Li X, Yang X, Maimaitijuma T et al (2019) Plant homeodomain finger protein 23 inhibits autophagy and promotes apoptosis of chondrocytes in osteoarthritis. Chin Med J (Engl) 132(21):2581–2587. https://doi.org/10.1097/cm9.0000000000000402
Guillén MI, Tofiño-Vian M, Silvestre A et al (2021) Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J Orthop Translat 30:61–69. https://doi.org/10.1016/j.jot.2021.08.003
Wang Y, Zhang H (2019) Regulation of Autophagy by mTOR Signaling Pathway. Adv Exp Med Biol 1206:67–83. https://doi.org/10.1007/978-981-15-0602-4_3
Pupyshev AB, Klyushnik TP, Akopyan AA et al (2022) Disaccharide trehalose in experimental therapies for neurodegenerative disorders: Molecular targets and translational potential. Pharmacol Res 183:106373. https://doi.org/10.1016/j.phrs.2022.106373
Deeks ED, Dhillon S (2010) Spotlight on strontium ranelate: in postmenopausal osteoporosis. Drugs Aging 27(9):771–773. https://doi.org/10.2165/11206440-000000000-00000
Han W, Fan S, Bai X et al (2017) Strontium ranelate, a promising disease modifying osteoarthritis drug. Expert Opin Investig Drugs 26(3):375–380. https://doi.org/10.1080/13543784.2017.1283403
Deng C, Zhu H, Li J et al (2018) Bioactive Scaffolds for Regeneration of Cartilage and Subchondral Bone Interface. Theranostics 8(7):1940–1955. https://doi.org/10.7150/thno.23674
Matyas JJ, O’Driscoll CM, Yu L et al (2017) Truncated TrkB.T1-Mediated Astrocyte Dysfunction Contributes to Impaired Motor Function and Neuropathic Pain after Spinal Cord Injury. J Neurosci 37(14):3956–3971. https://doi.org/10.1523/jneurosci.3353-16.2017
Kato S, Wakabayashi H, Nakagawa T et al (2020) Teriparatide improves pain-related behavior and prevents bone loss in ovariectomized mice. J Orthop Surg (Hong Kong) 28(1):2309499019893194. https://doi.org/10.1177/2309499019893194
Glasson SS, Chambers MG, Van Den Berg WB et al (2010) The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil 18 Suppl 3S17–23. https://doi.org/10.1016/j.joca.2010.05.025
Liu Y, Yang Y, Lin Y et al (2022) N(6) -methyladenosine-modified circRNA RERE modulates osteoarthritis by regulating β-catenin ubiquitination and degradation. https://doi.org/10.1111/cpr.13297. Cell Prolif:e13297
Isvoranu G, Manole E, Neagu M (2021) Gait Analysis Using Animal Models of Peripheral Nerve and Spinal Cord Injuries. Biomedicines 9(8). https://doi.org/10.3390/biomedicines9081050
Walter J, Kovalenko O, Younsi A et al (2020) The CatWalk XT® is a valid tool for objective assessment of motor function in the acute phase after controlled cortical impact in mice. Behav Brain Res 392:112680. https://doi.org/10.1016/j.bbr.2020.112680
Fouasson-Chailloux A, Dauty M, Bodic B et al (2021) Posttraumatic Osteoarthritis Damage in Mice: From Histological and Micro-Computed Tomodensitometric Changes to Gait Disturbance. Cartilage 13(2suppl):1478s–1489s. https://doi.org/10.1177/19476035211053821
Tschon M, Salamanna F, Martini L et al (2020) Boosting the Intra-Articular Efficacy of Low Dose Corticosteroid through a Biopolymeric Matrix: An In Vivo Model of Osteoarthritis. Cells 9(7). https://doi.org/10.3390/cells9071571
Parvathy SS, Masocha W (2013) Gait analysis of C57BL/6 mice with complete Freund’s adjuvant-induced arthritis using the CatWalk system. BMC Musculoskelet Disord 14:14. https://doi.org/10.1186/1471-2474-14-14
Torzilli PA, Allen SN (2022) Effect of Articular Surface Compression on Cartilage Extracellular Matrix Deformation. J Biomech Eng 144(9). https://doi.org/10.1115/1.4054108
Chen Z, Zhao C, Liu P et al (2021) Anti-Apoptosis and Autophagy Effects of Melatonin Protect Rat Chondrocytes against Oxidative Stress via Regulation of AMPK/Foxo3 Pathways. Cartilage 13(2suppl):1041s–1053s. https://doi.org/10.1177/19476035211038748
Hao R, Li M, Li F et al (2022) Protective effects of the phenolic compounds from mung bean hull against H(2)O(2)-induced skin aging through alleviating oxidative injury and autophagy in HaCaT cells and HSF cells. Sci Total Environ 841:156669. https://doi.org/10.1016/j.scitotenv.2022.156669
Rodrigues TA, Freire AO, Bonfim BF et al (2018) Strontium ranelate as a possible disease-modifying osteoarthritis drug: a systematic review. Braz J Med Biol Res 51(8):e7440. https://doi.org/10.1590/1414-431x20187440
Guo S, Yu D, Xiao X et al (2020) A vessel subtype beneficial for osteogenesis enhanced by strontium-doped sodium titanate nanorods by modulating macrophage polarization. J Mater Chem B 8(28):6048–6058. https://doi.org/10.1039/d0tb00282h
Okita N, Honda Y, Kishimoto N et al (2015) Supplementation of strontium to a chondrogenic medium promotes chondrogenic differentiation of human dedifferentiated fat cells. Tissue Eng Part A 21(9–10):1695–1704. https://doi.org/10.1089/ten.TEA.2014.0282
Cheng Y, Huang L, Wang Y et al (2019) Strontium promotes osteogenic differentiation by activating autophagy via the the AMPK/mTOR signaling pathway in MC3T3–E1 cells. Int J Mol Med 44(2):652–660. https://doi.org/10.3892/ijmm.2019.4216
Cai Z, Li Y, Song W et al (2021) Anti-Inflammatory and Prochondrogenic In Situ-Formed Injectable Hydrogel Crosslinked by Strontium-Doped Bioglass for Cartilage Regeneration. ACS Appl Mater Interfaces 13(50):59772–59786. https://doi.org/10.1021/acsami.1c20565
Li Y, Chen M, Yan J et al (2021) Tannic acid/Sr(2+)-coated silk/graphene oxide-based meniscus scaffold with anti-inflammatory and anti-ROS functions for cartilage protection and delaying osteoarthritis. Acta Biomater 126:119–131. https://doi.org/10.1016/j.actbio.2021.02.046
Liu S, Cao C, Zhang Y et al (2019) PI3K/Akt inhibitor partly decreases TNF-α-induced activation of fibroblast-like synoviocytes in osteoarthritis. J Orthop Surg Res 14(1):425. https://doi.org/10.1186/s13018-019-1394-4
Li T, He H, Yang Z et al (2021) Strontium-doped gelatin scaffolds promote M2 macrophage switch and angiogenesis through modulating the polarization of neutrophils. Biomater Sci 9(8):2931–2946. https://doi.org/10.1039/d0bm02126a
Chen J, Long F (2015) mTORC1 Signaling Promotes Osteoblast Differentiation from Preosteoblasts. PLoS ONE 10(6):e0130627. https://doi.org/10.1371/journal.pone.0130627
Zhang J, Huang L, Shi X et al (2020) Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging 12(23):24270–24287. https://doi.org/10.18632/aging.202143
Yan R, Li J, Wu Q et al (2022) Trace Element-Augmented Titanium Implant With Targeted Angiogenesis and Enhanced Osseointegration in Osteoporotic Rats. Front Chem 10:839062. https://doi.org/10.3389/fchem.2022.839062
Salamanna F, Giavaresi G, Parrilli A et al (2019) Antiresorptive properties of strontium substituted and alendronate functionalized hydroxyapatite nanocrystals in an ovariectomized rat spinal arthrodesis model. Mater Sci Eng C Mater Biol Appl 95:355–362. https://doi.org/10.1016/j.msec.2017.11.016
Bonnevier J, Malmqvist U, Sonntag D et al (2002) Sustained norepinephrine contraction in the rat portal vein is lost when Ca(2+) is replaced with Sr(2+). Am J Physiol Cell Physiol 282(4):C845–852. https://doi.org/10.1152/ajpcell.00191.2001
Pors Nielsen S (2004) The biological role of strontium. Bone 35(3):583–588. https://doi.org/10.1016/j.bone.2004.04.026
Borciani G, Ciapetti G, Vitale-Brovarone C et al (2022) Strontium Functionalization of Biomaterials for Bone Tissue Engineering Purposes: A Biological Point of View. Mater (Basel) 15(5). https://doi.org/10.3390/ma15051724
Acknowledgements
This study was supported by the Military Medical Innovation Engineering Project (no.17cxz004). The funder had no role in study design, data collection, data analysis, interpretation, or writing the report.
Author information
Authors and Affiliations
Contributions
Liao and Shangguan designed the study; Binghui Liao, Ming Ding, Yingchun Wang, and Hu Xu performed the experiments; Binghui Liao and Hu Xu analyzed the data; and Binghui Liao and Lei Shangguan wrote the paper. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosures
All authors state that they have no conflict of interest and nothing to disclose.
Ethical approval
This study was not a clinical trial, and did not involve human participants. All animal experimental procedures were approved by the Animal Research Committee of the Air Force Military Medical University, People’s Republic of China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, B., Ding, M., Wang, Y. et al. Strontium ion attenuates osteoarthritis through inhibiting senescence and enhancing autophagy in fibroblast-like synoviocytes. Mol Biol Rep 50, 1437–1446 (2023). https://doi.org/10.1007/s11033-022-08112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08112-7