Abstract
Background
Fibroblast growth factors (FGFs) are key factors affecting diabetic wound healing. However, the FGF family’s expression patterns in skin and wounds influenced by both diabetes and sex are still unknown.
Methods and results
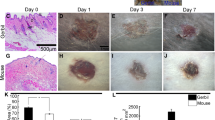
In this study, normal and Streptozotocin (STZ)-induced type 1 diabetic C57BL/6J male and female mice were used to study the FGF family’s expression in non-wound skin and wounds. We found that the expression patterns of Fgfs were affected by sex in both normal and diabetic animals during wound healing. In normal control mice, sex difference had a limited effect on basal skin Fgf expressions. However, it significantly influenced Fgf expressions in wounds. Type 1 diabetes reduced basal and wound-induced skin Fgf expressions. Female mice had far lower wound-induced skin Fgf expressions in diabetic mice. In addition, sex differently influenced Fibroblast growth factors receptor (Fgfr) expression patterns of non-wound skin and wounds in both normal and diabetic mice. Moreover, female mice had a lower relative level of Fibronectin leucine-rich repeat transmembrane protein 2 (FLRT2) — a FGFR activation marker gene — in wound and blood plasma. Correspondingly, the wound areas of female animals were larger than that of male animals in the early stage of wound healing (less than 3-day injury).
Conclusion
Our research shows that the FGF family have different expression patterns in normal and diabetic wound healing in mice of different sex. Additionally, we also provide the signatures of individual FGFs in diabetic wound healing, which deserve further investigation.






Similar content being viewed by others
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FGFs:
-
fibroblast growth factors
- FGFR:
-
fibroblast growth factor receptor
- FLRT:
-
fibronectin-leucine-rich transmembrane
- GTEx:
-
Genotype-Tissue Expression
- HRP:
-
Horseradish Peroxidase
- OD:
-
Optical Density
- STZ:
-
Streptozotocin
- TBP:
-
TATA-box binding protein
- T1D:
-
type 1 diabetes.
References
Chang M, Nguyen TT (2021) Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res 54(5):1080–1093
Centers for Disease Control and Prevention. National Diabetes Statistics Report website. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed [2022]
Cho H, Blatchley MR, Duh EJ, Gerecht S (2019) Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev 146:267–288
Shi M, Zhang H, Song T, Liu X, Gao Y, Zhou J, Li Y (2019) Sustainable dual release of antibiotic and growth factor from pH-Responsive uniform Alginate Composite Microparticles to enhance Wound Healing. ACS Appl Mater Interfaces 11(25):22730–22744
Xiong Y, Chen L, Liu P, Yu T, Lin C, Yan C, Hu Y, Zhou W, Sun Y, Panayi AC, Cao F, Xue H, Hu L, Lin Z, Xie X, Xiao X, Feng Q, Mi B, Liu G (2022) All-in-One: multifunctional hydrogel accelerates oxidative Diabetic Wound Healing through timed-release of exosome and fibroblast growth factor. Small 18(1):e2104229
Tuzon CT, Rigueur D, Merrill AE (2019) Nuclear fibroblast growth factor receptor signaling in skeletal development and disease. Curr Osteoporos Rep 17(3):138–146
Jia WH, Wang NQ, Yin L, Chen X, Hou BY, Wang JH, Qiang GF, Chan CB, Yang XY, Du GH (2020) Effects of fasting on the expression pattern of FGFs in different skeletal muscle fibre types and sexes in mice. Biol Sex Differ 11(1):9
Degirolamo C, Sabbà C, Moschetta A (2016) Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 15(1):51–69
Liu Y, Liu Y, Deng J, Li W, Nie X (2021) Fibroblast growth factor in Diabetic Foot Ulcer: progress and therapeutic prospects. Front Endocrinol (Lausanne) 12:744868
Komi-Kuramochi A, Kawano M, Oda Y, Asada M, Suzuki M, Oki J, Imamura T (2005) Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. J Endocrinol 186(2):273–289
Manurung D, Ilyas R, Hutahaean S, Rosidah S, Situmorang R PC (2021) Diabetic Wound Healing in FGF expression by Nano Herbal of Rhodomyrtus tomentosa L. and Zanthoxylum acanthopodium fruits. Pak J Biol Sci 24(3):401–408
Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L (2011) Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re-epithelialization, contraction, and angiogenesis. Ann Surg 254(6):1066–1074
Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, Sato K, Matsushita T, Nakamura K (2010) A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo-controlled trial. J Bone Miner Res 25(12):2735–2743
Xiaolan X, Wujie G, Xiaoyan T, Wenlai C (2019) A combination of ultrasonic debridement and Shenghong wet dressing in patients with chronic ulcers of the lower limbs. J Int Med Res 47(10):4656–4663
Korsensky L, Ron D (2016) Regulation of FGF signaling: recent insights from studying positive and negative modulators. Semin Cell Dev Biol 53:101–114
Karaulanov EE, Böttcher RT, Niehrs C (2006) A role for fibronectin-leucine-rich transmembrane cell-surface proteins in homotypic cell adhesion. EMBO Rep 7(3):283–290
Wei K, Xu Y, Tse H, Manolson MF, Gong SG (2011) Mouse FLRT2 interacts with the extracellular and intracellular regions of FGFR2. J Dent Res 90(10):1234–1239
Xu J, Liu X, Zhao F, Zhang Y, Wang Z (2020) HIF1α overexpression enhances diabetic wound closure in high glucose and low oxygen conditions by promoting adipose-derived stem cell paracrine function and survival. Stem Cell Res Ther 11(1):148
Werner S, Breeden M, Hübner G, Greenhalgh DG, Longaker MT (1994) Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol 103(4):469–473
Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M, Meinus S, Reinecke F, Möhlig M, Weickert MO, Clemenz M, Pfeiffer AF, Kintscher U, Spuler S, Spranger J (2009) Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 58(7):1532–1538
Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, Veves A (2012) Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 61(11):2937–2947
Kautzky-Willer A, Harreiter J, Pacini G (2016) Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes Mellitus. Endocr Rev 37(3):278–316
Dinh T, Veves A (2008) The influence of gender as a risk factor in diabetic foot ulceration. Wounds 20(5):127–131
Jain AK, Velazquez-Ramirez G, Goodney PP, Edwards MS, Corriere MA (2011) Gender-based analysis of perioperative outcomes associated with lower extremity bypass. Am Surg 77(7):844–849
Gilliver SC, Ashcroft GS (2007) Sex steroids and cutaneous wound healing: the contrasting influences of estrogens and androgens. Climacteric 10(4):276–288
Arnaoutakis DJ, Scully RE, Sharma G, Shah SK, Ozaki CK, Belkin M, Nguyen LL (2017) Impact of body mass index and gender on wound complications after lower extremity arterial surgery. J Vasc Surg 65(6):1713–1718e1
Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB, Boulton AJ (2001) The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med 18(2):133–138
El Yazidi I, Renaud F, Laurent M, Courtois Y, Boilly-Marer Y (1998) Production and oestrogen regulation of FGF1 in normal and cancer breast cells. Biochim Biophys Acta 1403(2):127–140
Bär L, Wächter K, Wege N, Navarrete Santos A, Simm A, Föller M (2017) Advanced glycation end products stimulate gene expression of fibroblast growth factor 23. Mol Nutr Food Res 61(8). doi: https://doi.org/10.1002/mnfr.201601019
Fontaine DA, Davis DB (2016) Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse Consortium. Diabetes 65(1):25–33
Hayashi K, Kojima R, Ito M (2006) Strain differences in the diabetogenic activity of streptozotocin in mice. Biol Pharm Bull 29(6):1110–1119
Eleazu CO, Eleazu KC, Chukwuma S, Essien UN (2013) Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord 12(1):60
Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ (2011) Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. J Invest Dermatol 131(2):518–528
Kastenmayer RJ, Fain MA, Perdue KA (2006) A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45(6):8–12
Tkalcević VI, Cuzić S, Parnham MJ, Pasalić I, Brajsa K (2009) Differential evaluation of excisional non-occluded wound healing in db/db mice. Toxicol Pathol 37(2):183–192
Liu Y, Liu Y, He W, Mu X, Wu X, Deng J, Nie X (2022) Fibroblasts: immunomodulatory factors in refractory diabetic wound healing. Front Immunol 13:918223
Duraisamy Y, Slevin M, Smith N, Bailey J, Zweit J, Smith C, Ahmed N, Gaffney J (2001) Effect of glycation on basic fibroblast growth factor induced angiogenesis and activation of associated signal transduction pathways in vascular endothelial cells: possible relevance to wound healing in diabetes. Angiogenesis 4(4):277–288
Yang XY, Tse M, Hu X, Jia WH, Du GH, Chan CB (2018) Interaction of CREB and PGC-1alpha induces fibronectin type III domain-containing protein 5 expression in C2C12 myotubes. Cell Physiol Biochem 50(4):1574–1584
Jia WH, Wang NQ, Yin L, Chen X, Hou BY, Qiang GF, Chan CB, Yang XY, Du GH (2019) Effect of skeletal muscle phenotype and gender on fasting-induced myokine expression in mice. Biochem Biophys Res Commun 514(2):407–414
Qiang G, Kong HW, Fang D, McCann M, Yang X, Du G, Blüher M, Zhu J, Liew CW (2016) The obesity-induced transcriptional regulator TRIP-Br2 mediates visceral fat endoplasmic reticulum stress-induced inflammation. Nat Commun 7:11378
Nam K, Lee KW, Chung O, Yim HS, Cha SS, Lee SW, Jun J, Cho YS, Bhak J, Magalhães JP, Lee JH, Jeong JY (2017) Analysis of the FGF gene family provides insights into aquatic adaptation in cetaceans. Sci Rep 7:40233
Manzano-Núñez F, Arámbul-Anthony MJ, Galán Albiñana A, Leal Tassias A, Acosta Umanzor C, Borreda Gascó I, Herrera A, Forteza Vila J, Burks DJ, Noon LA (2019) Insulin resistance disrupts epithelial repair and niche-progenitor fgf signaling during chronic liver injury. PLoS Biol 17(1):e2006972
Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM (2001) Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104(6):875–889
Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, Tate CR, Hevener AL, Najjar SM, Leloup C, Mauvais-Jarvis F (2014) Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab 3(2):177–190
Karpen CW, Spanheimer RG, Randolph AL, Lowe WL Jr (1992) Tissue-specific regulation of basic fibroblast growth factor mRNA levels by diabetes. Diabetes 41(2):222–226
Böttcher RT, Pollet N, Delius H, Niehrs C (2004) The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat Cell Biol 6(1):38–44
Haines BP, Wheldon LM, Summerbell D, Heath JK, Rigby PW (2006) Regulated expression of FLRT genes implies a functional role in the regulation of FGF signalling during mouse development. Dev Biol 297(1):14–25
Dai S, Zhou Z, Chen Z, Xu G, Chen Y (2019) Fibroblast growth factor receptors (FGFRs): structures and small molecule inhibitors. Cells 8(6):614
Hui Q, Jin Z, Li X, Liu C, Wang X (2018) FGF family: from Drug Development to Clinical Application. Int J Mol Sci 19(7):1875
Acknowledgements
This work was supported by Beijing Municipal Natural Science Foundation (7222119), the CAMS Innovation Fund (2021-1-I2M-029, 2016-I2M-3-007), the China’s National Key R&D Programs (2017YFE0112900, 2018ZX09711001-003-005), and the NSFC (82070877, 81770847, and 81470159).
Author information
Authors and Affiliations
Contributions
DGH, YXY and QGF contributed to the study conception and design. WNQ, JWH, YL, LMD and SJM contributed to the acquisition of data. HBY, LZ and WNQ contributed to the analysis of data. WNQ and YXY wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All the animal experimental procedures were in compliance with the guidelines and granted by the Animal Care and Use Committee (Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Nq., Jia, Wh., Yin, L. et al. Sex difference on fibroblast growth factors (FGFs) expression in skin and wound of streptozotocin(STZ)-induced type 1 diabetic mice. Mol Biol Rep 50, 1981–1991 (2023). https://doi.org/10.1007/s11033-022-08094-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08094-6