Abstract
Aims
The purpose of this study was to investigate the mechanism of mifepristone serves as an anti-implantation contraceptive drug on aquaporins 1 (AQP1) expression.
Methods
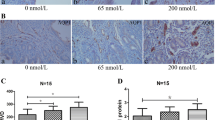
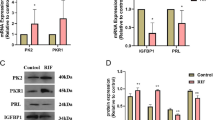
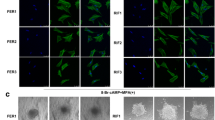
Human umbilical vein endothelial cells (HUVECs) were used to detect the effects of different concentrations of mifepristone (0, 0.065, 0.2, and 1 μmol/L) on the activity of angiogenesis and AQP1 expression. The expression of AQP1 was tested by the real-time PCR. The angiogenesis and penetration function of HUVECs was investigated by Matrigel lumen formation and trans-well assay, respectively.
Results
The expression of AQP1, angiogenesis and cell permeability were significantly higher than control groups in HUVECs treatment with mifepristone at 1 μmol/L for 12 h. Estrogen and progesterone decreased the up-regulation of AQP1 and cell permeability, not angiogenesis, induced by mifepristone. Mifepristone increased protein levels of p-ERK, not p-p38 or p-JNK, and pre-treatment with ERK MAPK-specific inhibitor significantly inhibited the up-regulation of AQP1 mRNA expression, angiogenesis and cell permeability induced by mifepristone. si-AQP1 significantly reduced the up-regulation of angiogenesis, cell permeability and p-ERK/ERK ratio expression induced by mifepristone treatment. Overexpression of AQP1 enhanced the increase of expression ratio of p-ERK/ERK induced by mifepristone.
Conclusions
Low-dose mifepristone increased cell permeability, angiogenesis and AQP1 expression, which was involved in MAPK pathways. This provides new insights into the molecular mechanism of mifepristone serves as an anti-implantation contraceptive drug.





Similar content being viewed by others
References
Navot D, Bergh P (1991) Preparation of the human endometrium for implantation. Ann N Y Acad Sci 622:212–219
Benga G (2012) The first discovered water channel protein, later called aquaporin 1: molecular characteristics, functions and medical implications. Mol Aspects Med 33:518–534
Zhang D, Tan YJ, Qu F, Sheng JZ, Huang HF (2012) Functions of water channels in male and female reproductive systems. Mol Aspects Med 33:676–690
Feng C, Sun CC, Wang TT, He RH, Sheng JZ, Huang HF (2008) Decreased expression of endometrial vessel AQP1 and endometrial epithelium AQP2 related to anovulatory uterine bleeding in premenopausal women. Menopause 15:648–654
Mullertz KM, Strom C, Trautner S, Amtorp O, Nielsen S, Christensen S, Haunso S, Jonassen TE (2011) Downregulation of aquaporin-1 in alveolar microvessels in lungs adapted to chronic heart failure. Lung 189:157–166
Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S (2002) Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer 87:621–623
Huebert RC, Vasdev MM, Shergill U, Das A, Huang BQ, Charlton MR, LaRusso NF, Shah VH (2010) Aquaporin-1 facilitates angiogenic invasion in the pathological neovasculature that accompanies cirrhosis. Hepatology 52:238–248
Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS (2005) Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434:786–792
Huebert RC, Jagavelu K, Hendrickson HI, Vasdev MM, Arab JP, Splinter PL, Trussoni CE, Larusso NF, Shah VH (2011) Aquaporin-1 promotes angiogenesis, fibrosis, and portal hypertension through mechanisms dependent on osmotically sensitive microRNAs. Am J Pathol 179:1851–1860
Zhao W, Shen H, Yuan F, Li G, Sun Y, Shi Z, Zhang Y, Wang Z (2009) Induction stage-dependent expression of vascular endothelial growth factor and aquaporin-1 in diethylstilbestrol-treated rat pituitary. Eur J Histochem 53:53–60
Lindsay LA, Murphy CR (2004) Redistribution of aquaporins in uterine epithelial cells at the time of implantation in the rat. Acta Histochem 106:299–307
Comparison of three single doses of mifepristone as emergency contraception: a randomised trial. Task Force on Postovulatory Methods of Fertility Regulation. Lancet 1999, 353:697–702
Chen Y, Wang W, Zhuang Y, Chen X, Huang L (2011) Effects of low-dose mifepristone administration in two different 14-day regimens on the menstrual cycle and endometrial development: a randomized controlled trial. Contraception 84:64–70
Zhou F, Liang Y, Qian ZD, Zhuang YL, Chen YZ, Lv BJ, Zhou CY, Chen XD, Huang LL (2013) Low-dose mifepristone increases uterine expression of aquaporin 1/aquaporin 2 at the time of implantation. Contraception 87:844–849
Zhou F, Qian Z, Huang L (2019) Low-dose mifepristone increased angiogenesis in a manner involving AQP1. Arch Gynecol Obstet 299:579–584
Zhang G, Han H, Zhuge Z, Dong F, Jiang S, Wang W, Guimaraes DD, Schiffer TA, Lai EY, Ribeiro-Antonino-Carvalho LR, Lucena RB, Braga VA, Weitzberg E, Lundberg JO, Carlstrom M (2021) Renovascular effects of inorganic nitrate following ischemia-reperfusion of the kidney. Redox Biol 39:101836
Zhang G, Wang Q, Wang W, Yu M, Zhang S, Xu N, Zhou S, Cao X, Fu X, Ma Z, Liu R, Mao J, Lai EY (2018) Tempol protects against acute renal injury by regulating PI3K/Akt/mTOR and GSK3beta signaling cascades and afferent arteriolar activity. Kidney Blood Press Res 43:904–913
Kim Y, Lee S, Zhang H, Lee S, Kim H, Kim Y, Won MH, Kim YM, Kwon YG (2020) CLEC14A deficiency exacerbates neuronal loss by increasing blood-brain barrier permeability and inflammation. J Neuroinflammation 17:48
Tinsley JH, Teasdale NR, Yuan SY (2004) Involvement of PKCdelta and PKD in pulmonary microvascular endothelial cell hyperpermeability. Am J Physiol Cell Physiol 286:C105–C111
Croxatto HB, Kovacs L, Massai R, Resch BA, Fuentealba B, Salvatierra AM, Croxatto HD, Zalanyi S, Viski S, Krenacs L (1998) Effects of long-term low-dose mifepristone on reproductive function in women. Hum Reprod 13:793–798
Ghosh D, Sengupta J (1993) Anti-nidatory effect of a single, early post-ovulatory administration of mifepristone (RU 486) in the rhesus monkey. Hum Reprod 8:552–558
Mahajan DK, London SN (1997) Mifepristone (RU486): a review. Fertil Steril 68:967–976
Hildenbrand A, Stavreus-Evers A, Lalitkumar PG, Nielsen S, Mints M, Gemzell-Danielsson K (2008) Aquaporin 1 is expressed in the human endometrium during normal cycle and increases after mifepristone treatment. Int J Mol Med 22:49–53
Yang M, Gao F, Liu H, Yu WH, Zhuo F, Qiu GP, Ran JH, Sun SQ (2013) Hyperosmotic induction of aquaporin expression in rat astrocytes through a different MAPK pathway. J Cell Biochem 114:111–119
Liu L, Xie C (2011) Effects of downregulation of aquaporin1 by peptidoglycan and lipopolysaccharide via MAPK pathways in MeT-5A cells. Lung 189:331–340
Zou LB, Shi S, Zhang RJ, Wang TT, Tan YJ, Zhang D, Fei XY, Ding GL, Gao Q, Chen C, Hu XL, Huang HF, Sheng JZ (2013) Aquaporin-1 plays a crucial role in estrogen-induced tubulogenesis of vascular endothelial cells. J Clin Endocrinol Metab 98:E672–E682
Acknowledgements
Not applicable.
Funding
Funding was provided by National Natural Science Foundation of China (Grant No.: 81701502).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors of this manuscript state that they do not have any conflict of interests and nothing to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Kang, Y., Jiang, Y. et al. Mifepristone increases AQP1 mRNA expression, angiogenesis, and cell permeability through the ERK MAPK pathway. Mol Biol Rep 50, 1069–1077 (2023). https://doi.org/10.1007/s11033-022-08082-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08082-w