Abstract
Background
Copper is both a nutrient essential for plant growth and a pollutant. In recent decades, with the rapid development of industrial and agricultural production, copper has been used more and more widely, and its consumption has also increased rapidly. Excessive soil copper contents induce phytotoxicity, affecting plant growth, development and yields. Moreover, copper can accumulate in crops and enter the food chain through enrichment, harming human health.
Methods and results
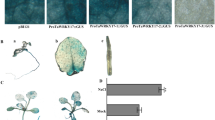
In this study, Arabidopsis wild-type (WT) and Zostera japonica 14-3-3 gene ZjGRF1 overexpression lines were used to explore the physiological function and molecular mechanism of ZjGRF1 in Arabidopsis in the copper stress response. Under copper stress, compared with WT plants, transgenic ZjGRF1 Arabidopsis plants exhibited less inhibition of root growth and development and had higher fresh weights. Under copper stress, the soluble sugar and soluble protein contents in transgenic ZjGRF1 Arabidopsis plants were significantly higher than those in WT plants, while the superoxide dismutase (SOD), peroxidase and catalase (CAT) activities were significantly higher than those in WT plants. Additionally, the malonaldehyde content of transgenic plants was significantly lower than that of WT plants. Furthermore, qRT-PCR results showed that under copper stress, the SOD, CAT1 and HMA5 expression levels in transgenic ZjGRF1 Arabidopsis plants were significantly higher than those in WT plants, while COPT1 expression was significantly lower than that in WT plants.
Conclusions
ZjGRF1 enhanced the copper stress resistance of Arabidopsis by maintaining high antioxidant enzyme activity, increasing copper efflux and reducing copper uptake under copper stress.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article or supplementary file. The nucleotide and deduced amino acid sequence data of ZjGRF1 and ZjGRF2 were submitted to GenBank (No. MW199706, MW206765).
Code Availability
Not applicable.
References
Brandt J et al (1992) A pathogen-induced gene of barley encodes a protein showing high similarity to a protein kinase regulator. Plant J 2(5):815–820
Lu G et al (1992) Brain proteins in plants: an Arabidopsis homolog to neurotransmitter pathway activators is part of a DNA binding complex. Proc Natl Acad Sci U S A 89(23):11490–11494
Hirsch S et al (1992) A plant homologue to mammalian brain 14-3-3 protein and protein kinase C inhibitor. FEBS Lett 296(2):222–224
DeLille JM, Sehnke PC, Ferl RJ (2001) The arabidopsis 14-3-3 family of signaling regulators. Plant Physiol 126(1):35–38
Rosenquist M et al (2001) Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant Physiol 127(1):142–149
Yao Y et al (2007) Molecular analysis and expression patterns of the 14-3-3 gene family from Oryza sativa. J Biochem Mol Biol 40(3):349–357
Li X, Dhaubhadel S (2011) Soybean 14-3-3 gene family: identification and molecular characterization. Planta 233(3):569–582
Yaffe MB et al (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91(7):961–971
Fu H, Subramanian RR, Masters SC (2000) 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol 40:617–647
Sumioka A et al (2005) Role of 14-3-3gamma in FE65-dependent gene transactivation mediated by the amyloid beta-protein precursor cytoplasmic fragment. J Biol Chem 280(51):42364–42374
ORTH RJ et al (2006) A Global Crisis for Seagrass Ecosystems. Bioscience 56(12):987–996
Iongh H et al (2007) A review of research on the interactions between dugongs (Dugong dugon Müller 1776) and intertidal seagrass beds in Indonesia. Hydrobiologia 591(1):73–83
Waycott M et al (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci U S A 106(30):12377–12381
Short FT et al (2011) Extinction risk assessment of the world’s seagrass species. Biol Conserv 144(7):1961–1971
Hyunchur et al (1998) Taxonomy and distribution of Zostera (Zosteraceae) in eastern Asia, with special reference to Korea. Aquat Bot 60(1):49–66
Prasad MNV, Strazlka K (1999) Impact of Heavy Metals on Photosynthesis. Heavy Metal Stress in Plants, : p.117–138
Maksymiec W, Wójcik M, Krupa Z (2007) Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 66(3):421–427
Macinnis-Ng C, Ralph PJ (2004) Variations in sensitivity to copper and zinc among three isolated populations of the seagrass, Zostera capricorni - ScienceDirect. J Exp Mar Biol Ecol 302(1):63–83
Aragones LV et al (2006) Dugong grazing and turtle cropping: grazing optimization in tropical seagrass systems? Oecologia 149(4):635–647
Chen S, Qiu G (2022) 14-3-3 gene of Zostera japonica ZjGRF1 participates in gibberellin signaling pathway. Mol Biol Rep
Chen S, Qiu G, Yang M (2019) SMRT sequencing of full-length transcriptome of seagrasses Zostera japonica. Sci Rep 9(1):14537
Sun BY et al (2010) Certain antioxidant enzymes and lipid peroxidation of radish (Raphanus sativus L.) as early warning biomarkers of soil copper exposure. J Hazard Mater 183(1–3):833–838
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53(372):1351–1365
Cho UH, Ji YS (2004) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and lipid peroxidation inArabidopsis thaliana. J Plant Biology 47(3):262–269
Skórzyńska-Polit E, Drkiewicz M, Krupa Z (2010) Lipid peroxidation and antioxidative response in Arabidopsis thaliana exposed to cadmium and copper. Acta Physiol Plant 32(1):169
Sancenón V et al (2004) The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J Biol Chem 279(15):15348–15355
Andrés-Colás N et al (2006) The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J 45(2):225–236
Burkhead JL et al (2010) Copper homeostasis. New Phytol 182(4):799–816
Ishida S et al (2004) Involvement of 14-3-3 signaling protein binding in the functional regulation of the transcriptional activator REPRESSION OF SHOOT GROWTH by gibberellins. Plant Cell 16(10):2641–2651
del Viso F, Casaretto JA, Quatrano RS (2007) 14-3-3 Proteins are components of the transcription complex of the ATEM1 promoter in Arabidopsis. Planta 227(1):167–175
Yin Y et al (2005) A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120(2):249–259
Solano R, Ecker JR (1998) Ethylene gas: perception, signaling and response. Curr Opin Plant Biol 1(5):393–398
Shin R et al (2011) 14-3-3 proteins fine-tune plant nutrient metabolism. FEBS Lett 585(1):143–147
Hayashi M et al (2011) Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol 52(7):1238–1248
Martin MH, Marschner H (1988) Mineral Nutrition Of Higher Plants. J Ecol 76(4):1250
Harrison MD, Jones CE, Dameron CT (1999) Copper chaperones: function, structure and copper-binding properties. J Biol Inorg Chem 4(2):145–153
Williams LE, Mills RF (2005) P(1B)-ATPases–an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci, 10(10): p.491–502
Shin LJ, Lo JC, Yeh KC (2012) Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiol 159(3):1099–1110
Acknowledgements
This study was funded by the Natural Science Foundation of Guangxi Province (2020GXNSFAA297067), the Research Fund Program of the Guangxi Key Lab of Mangrove Conservation and Utilization (GKLMC-21A01; GKLMC-20A04; GKLMC-20A01; 662), the National Natural Science Foundation of China (32170399) and the National Science & Technology Fundamental Resources Investigation Program of China (2019FY100604). These funding sources had no role in the study design, collection, analysis, interpretation of data and manuscript writing.
Funding
This study was funded by the Natural Science Foundation of Guangxi Province (2020GXNSFAA297067), the Research Fund Program of Guangxi Key Lab of Mangrove Conservation and Utilization (GKLMC-21A01; GKLMC-20A04; GKLMC-20A01), the National Natural Science Foundation of China (32170399) and the National Science & Technology Fundamental Resources Investigation Program of China (2019FY100604). These funding organizations had no role in the study design, collection, analysis, interpretation of data and manuscript writing.
Author information
Authors and Affiliations
Contributions
SC designed and conducted the experiment. GQ conducted field sampling and identification. SC and GQ wrote the manuscript. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Qiu, G. Overexpression of Zostera japonica 14-3-3 gene ZjGRF1 enhances the resistance of transgenic Arabidopsis to copper stress. Mol Biol Rep 49, 11635–11641 (2022). https://doi.org/10.1007/s11033-022-07915-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07915-y