Abstract
Background
Thymol (2-isopropyl-5-methylphenol) is a colorless crystalline derivative of cymene, that possesses pleotropic pharmacological properties, including analgesic, antibacterial, antispasmodic, and anti-inflammatory activities. Thymol has also been recognized for its beneficial effect as an anti-tumor agent, but the precise mechanism for this has not been fully elucidated. We aimed to identifying whether thymol exerts anti-cancer activity in human U-87 malignant glioblastoma (GB) cells (U-87).
Methods and Results
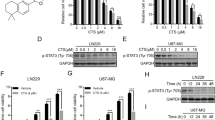
Cell viability and apoptosis was evaluated in U-87 cells treated with thymol at different concentrations. Reactive oxygen species (ROS) production, mRNA expressions of apoptosis-related genes and cell cycle characteristics were assessed. The cytotoxic activity of the co-exposure of thymol and temozolomide (TMZ) was also evaluated. The half-maximal inhibitory concentration (IC50) of thymol in the U-87 cells was 230 μM assessed at 24 h after exposure. Thymol did not exhibit any cytotoxic effects on normal L929 cells at this concentration. Thymol treatment increased the expression of Bax and p53, and also increased apoptotic cell death, and excessive generation of ROS. Moreover, the cytotoxic activity of thymol on the U-87 cells may be related to the arrest of the cell cycle at the G0/G1 interface. Combination therapy showed that the cytotoxic effects of thymol synergized with TMZ, and combined treatment had more cytotoxic potential compared to either of the agents alone.
Conclusions
Our data indicate the potential cytotoxic activities of thymol on U-87 cells. Further studies are required to evaluate the spectrum of the antitumor activity of thymol on GB cells.





Similar content being viewed by others
Data availability
The data supporting findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Ostrom QT et al (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17(Suppl 4):iv1–iv62
Ostrom QT et al (2020) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol 22(12 Suppl 2):iv1–iv96
Maghrouni A et al (2021) Targeting the PD-1/PD-L1 pathway in glioblastoma multiforme: preclinical evidence and clinical interventions. Int Immunopharmacol 93:107403
Sanati M et al (2022) How do phosphodiesterase-5 inhibitors affect cancer? A focus on glioblastoma multiforme. Pharmacol Rep. https://doi.org/10.1007/s43440-021-00349-6
Wu W et al (2021) Glioblastoma multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res 171:105780
Afshari AR et al (2021) Neurokinin-1 receptor (NK-1R) antagonists: potential targets in the treatment of glioblastoma multiforme. Curr Med Chem 28(24):4877–4892
Patel V, Shah J (2021) The current and future aspects of glioblastoma: immunotherapy a new hope? Eur J Neurosci 54(3):5120–5142
Afshari AR et al (2020) Modulation of calcium signaling in glioblastoma multiforme: a therapeutic promise for natural products. Mini Rev Med Chem 20(18):1879–1899
Bibak B et al (2022) Anticancer mechanisms of Berberine: a good choice for glioblastoma multiforme therapy. Curr Med Chem. https://doi.org/10.2174/0929867329666220224112811
Jalili-Nik M et al (2020) Zerumbone promotes cytotoxicity in human malignant glioblastoma cells through reactive oxygen species (ROS) generation. Oxidative Med Cell Longev. https://doi.org/10.1155/2020/3237983
Sahab-Negah S et al (2020) Curcumin loaded in niosomal nanoparticles improved the anti-tumor effects of free curcumin on glioblastoma stem-like cells: an in vitro study. Mol Neurobiol 57(8):3391–3411
Bagherniya M et al (2022) The effects of phytochemicals and herbal bio-active compounds on tumour necrosis factor-α in overweight and obese individuals: a clinical review. Inflammopharmacology. https://doi.org/10.1007/s10787-021-00902-y
Luthra R, Roy A (2022) Role of medicinal plants against neurodegenerative diseases. Curr Pharm Biotechnol 23(1):123–139
Jalili-Nik M et al (2018) Current status and future prospective of Curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J Cell Physiol 233(9):6337–6345
Soltani A et al (2019) 5′-Adenosine monophosphate-activated protein kinase: a potential target for disease prevention by curcumin. J Cell Physiol 234(3):2241–2251
Nagoor Meeran MF et al (2017) Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front Pharmacol 8:380
Yanishlieva NV et al (1999) Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem 64(1):59–66
Karpanen TJ et al (2008) Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J Antimicrob Chemother 62(5):1031–1036
Li Y et al (2017) Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem Biophys Res Commun 491(2):530–536
De La Chapa JJ et al (2018) Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J Oral Pathol Med 47(7):674–682
Lv R, Chen Z (2017) Thymol inhibits cell migration and invasion by downregulating the activation of PI3K/AKT and ERK pathways in human colon cancer cells. Trop J Pharm Res 16(12):2895–2901
Amirfakhri S, Salimi A, Fernandez N (2015) Effects of conditioned medium from breast cancer cells on Tlr2 expression in Nb4 cells. Asian Pac J Cancer Prev 16(18):8445–8450
Lee KP et al (2016) Regulation of C6 glioma cell migration by thymol. Oncol Lett 11(4):2619–2624
Soltani A et al (2021) Application of molecular docking for the development of improved HIV-1 reverse transcriptase inhibitors. Current Comput 17(4):538–549
International Organization for Standardization, 1992 ISO 10993–5. In Biological Evaluation of Medical Devices-Part 5. Tests for Cytotoxicity: In vitro Methods; ISO: Geneve, Switzerland
Schedle A et al (1995) Response of L-929 fibroblasts, human gingival fibroblasts, and human tissue mast cells to various metal cations. J Dent Res 74(8):1513–1520
Aydın E et al (2017) Anticancer, antioxidant and cytotoxic potential of thymol in vitro brain tumor cell model. Central Nerv Syst Agents Med Chem 17(2):116–122
Girola N et al (2015) Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem Biophys Res Commun 467(4):928–934
Özkan A, Erdoğan A (2011) A comparative evaluation of antioxidant and anticancer activity of essential oil from origanum onites (Lamiaceae) and its two major phenolic components. Turk J Biol 35(6):735–742
Elbe H et al (2020) Comparison of ultrastructural changes and the anticarcinogenic effects of thymol and carvacrol on ovarian cancer cells: which is more effective? Ultrastruct Pathol 44(2):193–202
Deb DD et al (2011) Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol Interact 193(1):97–106
Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R (2012) Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res 318(8):925–935
Huang H et al (2012) Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol Rep 27(6):2050–2056
Fiore G et al (2006) In vitro antiproliferative effect of six Salvia species on human tumor cell lines. Phytother Res 20(8):701–703
Eom KS et al (2010) Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction. Biol Pharm Bull 33(10):1644–1649
Gomez-Manzano C et al (1997) Characterization of p53 and p21 functional interactions in glioma cells en route to apoptosis. J Natl Cancer Inst 89(14):1036–1044
Chen J (2016) The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med 6(3):a026104–a026104
Riley T et al (2008) Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9(5):402–412
Daniele S et al (2018) Bax activation blocks self-renewal and induces apoptosis of human glioblastoma stem cells. ACS Chem Neurosci 9(1):85–99
Balan DJ et al (2021) Thymol induces mitochondrial pathway-mediated apoptosis via ROS generation, macromolecular damage and SOD diminution in A549 cells. Pharmacol Rep 73(1):240–254
Zeng Q et al (2020) Thymol isolated from thymus vulgaris l. Inhibits colorectal cancer cell growth and metastasis by suppressing the wnt/β-catenin pathway. Drug Des 14:2535
Jaafari A et al (2012) Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Rev Bras 22(3):534–540
Kang S-H et al (2016) Anticancer effect of thymol on AGS human gastric carcinoma cells. J Microbiol Biotechnol 26(1):28–37
Zhang WB et al (2010) Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem 285(52):40461–40471
Satooka H, Kubo I (2012) Effects of thymol on B16–F10 melanoma cells. J Agric Food Chem 60(10):2746–2752
Shettigar NB et al (2015) Thymol, a monoterpene phenolic derivative of cymene, abrogates mercury-induced oxidative stress resultant cytotoxicity and genotoxicity in hepatocarcinoma cells. Environ Toxicol 30(8):968–980
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5(5):415–418
Matés JM et al (2008) Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol 82(5):273–299
Soltani A et al (2019) The comparative study of the effects of Fe2O3 and TiO2 micro- and nanoparticles on oxidative states of lung and bone marrow tissues and colony stimulating factor secretion. J Cell Biochem 120(5):7573–7580
Yoshida K, Miki Y (2010) The cell death machinery governed by the p53 tumor suppressor in response to DNA damage. Cancer Sci 101(4):831–835
NavaneethaKrishnan S, Rosales JL, Lee K-Y (2019) ROS-mediated cancer cell killing through dietary phytochemicals. Oxidative Med Cell Longev. https://doi.org/10.1155/2019/9051542
Acknowledgements
We thankful from Mr. M. Jalili for kind cooperation. We appreciate the assistance of the Clinical Research Development Unit of Imam Reza hospital and Akbar Hospital, Mashhad University of Medical Sciences, in conducting this research.
Funding
This study supported by Vice-Chancellor for Research and Technology, Mashhad University of Medical Sciences, Iran (code: 4001891).
Author information
Authors and Affiliations
Contributions
AB: Methodology, Conceptualization, Formal analysis, Validation, Funding acquisition, Writing—original draft. FQ: Formal analysis, Validation, Writing—review & editing. NH: Methodology, Formal analysis, Validation. AS: Resources, Supervision, Writing—review & editing. SA: Methodology, Formal analysis, Validation. FS: Validation, Writing—review & editing. AT: Resources, Supervision, Writing—review & editing. AA Methodology, Formal analysis, Validation. GF: Supervision, review & editing. All Authors read and confirmed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The study was approved by the Deputy of Research and Technology and Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.MEDICAL.REC.1401.184).
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qoorchi Moheb Seraj, F., Heravi-Faz, N., Soltani, A. et al. Thymol has anticancer effects in U-87 human malignant glioblastoma cells. Mol Biol Rep 49, 9623–9632 (2022). https://doi.org/10.1007/s11033-022-07867-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07867-3