Abstract
Background
Reprogramming in transcriptional regulation provides an effective tool for adjusting cellular metabolic activities. The strong methanol-inducible alcohol oxidase-1 promoter (pAOX1) is commonly used for heterologous gene expression in the yeast Pichia pastoris. Here, we present a novel Pichia pastoris strain engineered to co-express methanol-induced transcription factor 1 (Mit1) and the target protein. Mit1 upregulates pAOX1 in response to methanol.
Methods and Results
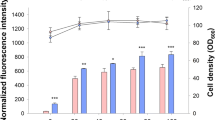
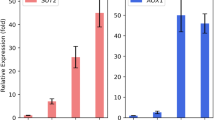
Two model proteins (VEGF and eGFP) have been used as the target proteins under the control of pAOX1. The sequence of Mit1 had obtained from the yeast genome and likewise cloned under the control of pAOX1. The results indicated a 1.9 and 2.2 fold increase in the detected VEGF and eGFP, respectively, when co-expressed with Mit1. Furthermore, the double-recombinant cells, containing Mit-1 and eGFP, produced 1.3 fold more eGFP when the methanol feeding concentration was doubled. The real-time PCR indicated a slight increase in the Mit1 expression, probably due to the negative regulatory feedback loop that exists for the intrinsic yeast Mit1. Overexpression of Mit1 also led to duplication of AOX1 enzyme activity, which may enhance the yeast cells’ capacity for methanol detoxification.
Conclusion
Overexpression of Mit1 could be considered a promising strategy for upregulation of target recombinant proteins in Pichia pastoris.
Graphical Abstract
Intracellular overexpression of Mit1 upregulates the heterologous target gene (eGFP) production, which is expressed under the control of pAOX1.





Similar content being viewed by others
References
Bonander N, Bill RM (2012) Optimizing yeast as a host for recombinant protein production (review). Methods Mol Biol 866:1–9. https://doi.org/10.1007/978-1-61779-770-5_1
Juturu V, Wu JC (2018) Heterologous protein expression in Pichia pastoris: latest research progress and applications. ChemBioChem 19:7–21. https://doi.org/10.1002/cbic.201700460
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98:5301–5317. https://doi.org/10.1007/s00253-014-5732-5
Guo C, Huang Y, Zheng H, Tang L, He J, Xiang L, Liu D, Jiang H (2012) Secretion and activity of antimicrobial peptide cecropin D expressed in Pichia pastoris. Exp Ther Med 4:1063–1068. https://doi.org/10.3892/etm.2012.719
Ergün BG, Berrios J, Binay B, Fickers P (2021) Recombinant protein production in Pichia pastoris: from transcriptionally redesigned strains to bioprocess optimization and metabolic modelling. FEMS Yeast Res 21:foab057. https://doi.org/10.1093/femsyr/foab057
Duman-Özdamar ZE, Binay B (2021) Production of industrial enzymes via Pichia pastoris as a cell factory in bioreactor: current status and future aspects. Protein J 40:367–376. https://doi.org/10.1007/s10930-021-09968-7
Hartner FS, Glieder A (2006) Regulation of methanol utilization pathway genes in yeasts. Microb Cell Fact 5:39. https://doi.org/10.1186/1475-2859-5-39
Vogl T, Sturmberger L, Fauland PC, Hyden P, Fischer JE, Schmid C, Thallinger GG, Geier M, Glieder A (2018) Methanol independent induction in Pichia pastoris by simple derepressed overexpression of single transcription factors. Biotechnol Bioeng 115:1037–1050. https://doi.org/10.1002/bit.26529
Wang X, Wang Q, Wang J, Bai P, Shi L, Shen W, Zhou M, Zhou X, Zhang Y, Cai M (2016) Mit1 transcription factor mediates methanol signaling and regulates the alcohol oxidase 1 (AOX1) promoter in Pichia pastoris. J Biol Chem 291:6245–6261. https://doi.org/10.1074/jbc.M115.692053
Wang J, Wang X, Shi L, Qi F, Zhang P, Zhang Y, Zhou X, Song Z, Cai M (2017) Methanol-independent protein expression by AOX1 promoter with trans-acting elements engineering and glucose-glycerol-shift induction in Pichia pastoris. Sci Rep 7:41850. https://doi.org/10.1038/srep41850
Garrigós-Martínez J, Nieto-Taype MA, Gasset-Franch A, Montesinos-Seguí JL, Garcia-Ortega X, Valero F (2019) Specific growth rate governs AOX1 gene expression, affecting the production kinetics of Pichia pastoris (Komagataella phaffii) PAOX1-driven recombinant producer strains with different target gene dosage. Microb Cell Factories 18:1–15. https://doi.org/10.1186/s12934-019-1240-8
Asareh SM, Savei T, Arjmand S, Siadat SO, Fatemi F, Pourmadadi M, Shayeh JS (2021) Expression of functional eGFP-fused antigen-binding fragment of ranibizumab in Pichia pastoris. BioImpacts 11:5. https://doi.org/10.34172/bi.2021.23219
Arjmand S, Tavasoli Z, Siadat SO, Saeidi B, Tavana H (2019) Enhancing chimeric hydrophobin II-vascular endothelial growth factor A165 expression in Pichia pastoris and its efficient purification using hydrophobin counterpart. Int J Biol Macromol 139:1028–1034. https://doi.org/10.1016/j.ijbiomac.2019.08.080
Mombeni M, Arjmand S, Siadat SO, Alizadeh H, Abbasi A (2020) pMOX: a new powerful promoter for recombinant protein production in yeast Pichia pastoris. Enz Microb Technol 109582. https://doi.org/10.1016/j.enzmictec.2020.109582
Arjmand S, Lotfi AS, Shamsara M, Mowla SJ (2013) Elevating the expression level of biologically active recombinant human alpha 1-antitrypsin in Pichia pastoris. Electron J Biotechnol 16. https://doi.org/10.2225/vol16-issue1-fulltext-4
Maceda-López LF, Villalpando-Aguilar JL, García-Hernández E, Ávila de Dios E, Andrade-Canto SB, Morán-Velázquez DC, Rodríguez-López L, Hernández-Díaz D, Chablé-Vega MA, Trejo L, Góngora-Castillo E (2021) Improved method for isolation of high-quality total RNA from Agave tequilana Weber roots. 3 Biotech 11:1–10. https://doi.org/10.1007/s13205-020-02620-8
Sukardi P, Rosita RE, Siregar AS, Januar CS, Harisam MT, Hidayat F, Bessho Y, Prayogo NA (2019) The effect of endosulfan (insecticide) on expression of vitelogenin gene in female silver sharkminnow (Osteochilus hasseltii CV). IOP Conference Series: Earth and Environmental Science. IOP Publishing. https://doi.org/10.1088/1755-1315/406/1/012032
Shen W, Kong C, Xue Y, Liu Y, Cai M, Zhang Y, Jiang T, Zhou X, Zhou M (2016) Kinase screening in Pichia pastoris identified promising targets involved in cell growth and alcohol oxidase 1 promoter (P AOX1) regulation. PLoS ONE 11:e0167766. https://doi.org/10.1371/journal.pone.0167766
Zhang H, Loovers HM, Xu LQ, Wang M, Rowling PJ, Itzhaki LS, Gong W, Zhou JM, Jones GW, Perrett S (2009) Alcohol oxidase (AOX1) from Pichia pastoris is a novel inhibitor of prion propagation and a potential ATPase. Mol Microbiol 71:702–716. https://doi.org/10.1111/j.1365-2958.2008.06557.x
Fischer JE, Glieder A (2019) Current advances in engineering tools for Pichia pastoris. Curr Opin Biotechnol 59:175–181. https://doi.org/10.1016/j.copbio.2019.06.002
Papadakis ED, Nicklin SA, Baker AH, White SJ (2004) Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther 4:89–113. https://doi.org/10.2174/1566523044578077
Cámara E, Monforte S, Albiol J, Ferrer P (2019) Deregulation of methanol metabolism reverts transcriptional limitations of recombinant Pichia pastoris (Komagataella spp) with multiple expression cassettes under control of the AOX1 promoter. Biotechnol Bioeng 116:1710–1720. https://doi.org/10.1002/bit.26947
Takagi S, Tsutsumi N, Terui Y, Kong X (2012) Method for methanol independent induction from methanol inducible promoters in Pichia. US8236528B2
Takagi S, Tsutsumi N, Terui Y, Kong X, Yurimoto H, Sakai Y (2019) Engineering the expression system for Komagataella phaffii (Pichia pastoris): an attempt to develop a methanol-free expression system. FEMS Yeast Res 19:foz059. https://doi.org/10.1093/femsyr/foz059
Chang CH, Hsiung HA, Hong KL, Huang CT (2018) Enhancing the efficiency of the Pichia pastoris AOX1 promoter via the synthetic positive feedback circuit of transcription factor Mxr1. BMC Biotechnol 18:1–10. https://doi.org/10.1186/s12896-018-0492-4
Yang Y, Zheng Y, Wang P, Li X, Zhan C, Linhardt RJ, Zhang F, Liu X, Zhan J, Bai Z (2020) Characterization and application of a putative transcription factor (SUT2) in Pichia pastoris. Mol Genet Genom 295:1295–1304. https://doi.org/10.1007/s00438-020-01697-3
Acknowledgement
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S.H.P. carried out experiments and contributed to the writing, S.O.R.S. supervised the study, S.A. planned, coordinated, and supervised the project and wrote the manuscript, M.K.S. assisted in conducting experiments.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All the authors have read and consented to submit the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haghighi Poodeh, S., Ranaei Siadat, S.O., Arjmand, S. et al. Improving AOX1 promoter efficiency by overexpression of Mit1 transcription factor. Mol Biol Rep 49, 9379–9386 (2022). https://doi.org/10.1007/s11033-022-07790-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07790-7