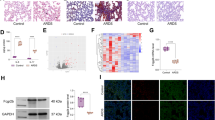
Abstract
Background
Lung injury caused by pulmonary inflammation is one of the main manifestations of respiratory diseases. Vasorin (VASN) is a cell-surface glycoprotein encoded by the VASN gene and is expressed in the lungs of developing mouse foetuses. Previous research has revealed that VASN is associated with many diseases. However, its exact function in the lungs and the underlying mechanism remain poorly understood.
Methods and results
To investigate the molecular mechanisms involved in lung disease caused by VASN deficiency, a VASN gene knockout (VASN−/−) model was established. The pathological changes in the lungs of VASN−/− mice were similar to those in a lung injury experimental mouse model. We further analysed the transcriptomes of the lungs of VASN−/− mice and wild-type mice. Genes in twenty-four signalling pathways were enriched in the lungs of VASN−/− mice, among which PPAR signalling pathway genes (3 genes, FABP4, Plin1, AdipoQ, were upregulated, while apoA5 was downregulated) were found to be closely related to lung injury. The most significantly changed lung injury-related gene, FABP4, was selected for further verification. The mRNA and protein levels of FABP4 were significantly increased in the lungs of VASN−/− mice, as were the mRNA and protein levels of the inflammatory factors IL-6, TNF-α and IL-1β.
Conclusions
We believe that these data provide molecular evidence for the regulatory role of VASN in inflammation in the context of lung injury.





Similar content being viewed by others
Data availability
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Goodman RB, Pugin J, Lee JS et al (2003) Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 14(6):523–535. https://doi.org/10.1016/s1359-6101(03)00059-5
Lin WC, Chen CW, Huang YW et al (2015) Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Sci Rep 5:12463. https://doi.org/10.1038/srep12463
Chen L, Yao J, Zhang S et al (2005) Slit-like 2, a novel zebrafish slit homologue that might involve in zebrafish central neural and vascular morphogenesis. Biochem Biophys Res Commun 336(1):364–371. https://doi.org/10.1016/j.bbrc.2005.08.071
Krautzberger AM, Kosiol B, Scholze M et al (2012) Expression of vasorin (VASN) during embryonic development of the mouse. Gene Expr Patterns 12(5–6):167–171. https://doi.org/10.1016/j.gep.2012.02.003
Ikeda Y, Imai Y, Kumagai H et al (2004) Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Proc Natl Acad Sci USA 101(29):10732–10737. https://doi.org/10.1073/pnas.0404117101
Rimon Dahari N, Heinemann Yerushalmi L, Hadas R et al (2018) Vasorin: a newly identified regulator of ovarian folliculogenesis. FASEB J 32(4):2124–2136. https://doi.org/10.1096/fj.201700057RRR
Malapeira J, Esselens C, Bech-Serra JJ et al (2011) ADAM17 (TACE) regulates TGFbeta signaling through the cleavage of vasorin. Oncogene 30(16):1912–1922. https://doi.org/10.1038/onc.2010.565
Sun J, Guo X, Ping Yu et al (2022) Vasorin deficiency leads to cardiac hypertrophy by targeting MYL7 in young mice. J Cell Mol Med 26(1):88–98. https://doi.org/10.1111/jcmm.17034
Choksi S, Lin Y, Pobezinskaya Y et al (2011) A HIF-1 target, ATIA, protects cells from apoptosis by modulating the mitochondrial thioredoxin, TRX2. Mol Cell 42(5):597–609. https://doi.org/10.1016/j.molcel.2011.03.030
Chen W, Wang Q, Xu X et al (2020) Vasorin/ATIA promotes cigarette smoke-induced transformation of human bronchial epithelial cells by suppressing autophagy-mediated apoptosis. Transl Oncol 13(1):32–41. https://doi.org/10.1016/j.tranon.2019.09.001
Furuhashi M, Tuncman G, Gorgun CZ et al (2007) Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 447(7147):959–965. https://doi.org/10.1038/nature05844
Rodríguez-Calvo R, Girona J, Alegret JM et al (2017) Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J Endocrinol 233(3):R173–R184. https://doi.org/10.1530/JOE-17-0031
Ghelfi E, Karaaslan C, Berkelhamer S et al (2011) Fatty acid-binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 45(3):550–556. https://doi.org/10.1165/rcmb.2010-0376OC
Shields KJ, Verdelis K, Passineau MJ et al (2016) Three-dimensional micro computed tomography analysis of the lung vasculature and differential adipose proteomics in the Sugen/hypoxia rat model of pulmonary arterial hypertension. Pulm Circ 6(4):586–596. https://doi.org/10.1086/688931
Gong Y, Yu Z, Gao Y et al (2018) FABP4 inhibitors suppress inflammation and oxidative stress in murine and cell models of acute lung injury. Biochem Biophys Res Commun 496(4):1115–1121. https://doi.org/10.1016/j.bbrc.2018.01.150
Park H, Park SE, Park C et al (2016) The relationship between serum fatty-acid binding protein 4 level and lung function in Korean subjects with normal ventilatory function. BMC Pulm Med. https://doi.org/10.1186/s12890-016-0190-8
Makowski L, Brittingham KC, Reynolds JM et al (2005) The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem 280(13):12888–12895. https://doi.org/10.1074/jbc.M413788200
Huang DW, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37(1):1–13. https://doi.org/10.1093/nar/gkn923
Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1):44–57. https://doi.org/10.1038/nprot.2008.211
Szklarczyk D, Franceschini A, Wyder S, et al (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43(Database issue): D447–D452. https://doi.org/10.1093/nar/gku1003
Dong W, He B, Qian H et al (2018) RAB26-dependent autophagy protects adherens junctional integrity in acute lung injury. Autophagy 14(10):1677–1692. https://doi.org/10.1080/15548627.2018.1476811
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Bonnet A, Chaussain C, Broutin I et al (2018) From vascular smooth muscle cells to folliculogenesis: what about vasorin? Front Med. https://doi.org/10.3389/fmed.2018.00335
Bi J, Cui R, Li Z et al (2017) Astaxanthin alleviated acute lung injury by inhibiting oxidative/nitrative stress and the inflammatory response in mice. Biomed Pharmacother 95:974–982. https://doi.org/10.1016/j.biopha.2017.09.012
Cornelio FD, Martins TM, Lemos DAE et al (2013) Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediat Inflamm 2013:164202. https://doi.org/10.1155/2013/164202
Xu M, Liu G, Jia Y et al (2018) Transplantation of human placenta mesenchymal stem cells reduces the level of inflammatory factors in lung tissues of mice with acute lung injury. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 34(2):105–109
Zhong WJ, Yang HH, Guan XX et al (2019) Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J Cell Physiol 234(4):4641–4654. https://doi.org/10.1002/jcp.27261
Baradaran RV, Rakhshandeh H, Raucci F et al (2019) Anti-inflammatory and anti-oxidant activity of portulaca oleracea extract on LPS-induced rat lung injury. Molecules. https://doi.org/10.3390/molecules24010139
Manicone AM (2009) Role of the pulmonary epithelium and inflammatory signals in acute lung injury. Expert Rev Clin Immunol 5(1):63–75. https://doi.org/10.1586/177666X.5.1.63
Spadaro S, Park M, Turrini C et al (2019) Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm (Lond) 16:1. https://doi.org/10.1186/s12950-018-0202-y
Hertzel AV, Bernlohr DA (2000) The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab 11(5):175–180. https://doi.org/10.1016/s1043-2760(00)00257-5
Acknowledgements
We thank Yuanjie Huang and Jiaquan Li (Institute of Life Sciences, Guangxi Medical University) for technical help with histology.
Funding
This study is supported by the Central Government Guides the Development of Local Science and Technology (GUIKEZY1949025 and GUIKEZY20198024).
Author information
Authors and Affiliations
Contributions
Conceptualization: XG, YO, and MH; investigation/data acquisition: XG, JS, JL, SZ, MZ, LY, XH, KX, SW, and ZM; writing and visualization: XG and MH; supervision and resource support: BH and JL; project administration and funding acquisition: YO and MH.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All animal experiments were approved by the local ethics committee for animal welfare (Guangxi Medical University, China, 201909027) and performed in accordance with the Association for Research statement on the use of animals in lung research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, X., Sun, J., Liang, J. et al. Vasorin contributes to lung injury via FABP4-mediated inflammation. Mol Biol Rep 49, 9335–9344 (2022). https://doi.org/10.1007/s11033-022-07780-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07780-9