Abstract
Background
Coronary artery disease (CAD), is the leading cause of mortality and morbidity worldwide. Tenascin-C (TNC) with high expression levels in inflammatory and cardiovascular diseases, leads to the rupture of atherosclerotic plaques. The origin of plaque destabilization can be associated to endothelial dysfunction. Given the high prevalence of CAD, finding valuable biomarkers for its early detection is of great interest. Using serum samples from patients with CAD and individuals without CAD, we assessed the efficacy of TNC expression levels in serum exosomes and during endothelial cell differentiation as a noninvasive biomarker of CAD.
Methods
TNC expression was analyzed using the RNA-sequencing data sets of 6 CAD and 6 normal samples of blood exosomes and endothelial differentiation transitions. Additionally, TNC expression was investigated in the serum samples of patients with CAD and individuals without CAD via qRT-PCR. ROC curve analysis was employed to test the suitability of TNC expression alterations as a CAD biomarker.
Results
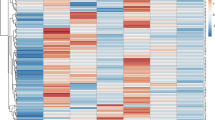
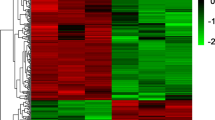
TNC exhibited higher expression in the exosomes of the CAD samples than in those of the non-CAD samples. During endothelial differentiation, TNC expression was upregulated and then consistently downregulated in mature endothelial cells. Moreover, TNC was significantly upregulated in the serum of the CAD group (P = 0.02), with an AUC of 0.744 for the expression level (95% confidence interval, 0.582 to 0.907; P = 0.011). Hence its expression level can be discriminated CAD from non-CAD samples.
Discussion
Our study is the first to confirm that altered TNC expression is associated with pathological CAD conditions in Iranian patients. The expression of TNC is involved in endothelial differentiation and CAD development. Accordingly, TNC can serve as a valuable noninvasive biomarker with potential application in CAD diagnosis.


Similar content being viewed by others
Data Availability
Data and material are available on request from the correspondence author.
References
Wilson PW et al (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97(18):1837–1847
Mackay J, Mensah GA, Greenlund K (2004) The atlas of heart disease and stroke. World Health Organization
Kinlay S, Libby P, Ganz P (2001) Endothelial function and coronary artery disease. Curr Opin Lipidol 12(4):383–389
Lüscher TF, Barton M (1997) Biology of the endothelium. Cli Cardiol 20:II-3–II-10
Gao W et al (2018) Tenascin C: a potential biomarker for predicting the severity of coronary atherosclerosis. J Atheroscler Thromb 42887
Watkins H, Farrall M (2006) Genetic susceptibility to coronary artery disease: from promise to progress. Nat Rev Genet 7(3):163–173
Medina-Leyte DJ et al (2021) Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci 22(8):3850
Bugiardini R et al (2004) Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation 109(21):2518–2523
Matsuzawa Y, Lerman A (2014) Endothelial dysfunction and coronary artery disease: assessment, prognosis and treatment. Coron Artery Dis 25(8):713
Wang S et al (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15(2):261–271
Furumoto T et al (2002) Association of cardiovascular risk factors and endothelial dysfunction in Japanese hypertensive patients: implications for early atherosclerosis. Hypertens Res 25(3):475–480
Zampetaki A et al (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circul Res 107(6):810–817
Gholipour A et al (2022) Bioinformatics Analysis to Find Novel Biomarkers for Coronary Heart Disease. Iran J Public Health 51(5):1152–1160
Barua RS et al (2002) Smoking is associated with altered endothelial-derived fibrinolytic and antithrombotic factors: an in vitro demonstration. Circulation 106(8):905–908
Barua RS et al (2003) Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation 107(18):2342–2347
Koskinas KC et al (2009) The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr Opin Cardiol 24(6):580–590
Gholipour A et al (2022) Downregulation of Talin-1 is associated with the increased expression of miR‐182‐5p and miR‐9‐5p in coronary artery disease. J Clin Lab Anal e24252
Mirzadeh Azad F et al (2020) Small molecules with big impacts on cardiovascular diseases. Biochem Genet 58(3):359–383
Midwood KS et al (2016) Tenascin-C at a glance. J Cell Sci 129(23):4321–4327
Midwood KS, Orend G (2009) The role of tenascin-C in tissue injury and tumorigenesis. J cell communication Signal 3(3):287–310
Imanaka-Yoshida K et al (2001) Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab Invest 81(7):1015–1024
Willems IE, Arends JW, Daemen MJ (1996) Tenascin and fibronectin expression in healing human myocardial scars. J Pathol 179(3):321–325
Imanaka-Yoshida K et al (2002) Tenascin‐C is a useful marker for disease activity in myocarditis. J Pathol 197(3):388–394
Jones PL, Cowan KN, Rabinovitch M (1997) Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 150(4):1349
Jones PL, Rabinovitch M (1996) Tenascin-C is induced with progressive pulmonary vascular disease in rats and is functionally related to increased smooth muscle cell proliferation. Circul Res 79(6):1131–1142
Midwood KS et al (2011) Advances in tenascin-C biology. Cell Mol Life Sci 68(19):3175–3199
Ballard VL et al (2006) Vascular tenascin-C regulates cardiac endothelial phenotype and neovascularization. FASEB J 20(6):717–719
Yamamoto K et al (2005) Tenascin-C is an essential factor for neointimal hyperplasia after aortotomy in mice. Cardiovascular Res 65(3):737–742
Nishioka T et al (2010) Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 298(3):H1072–H1078
Pak HN et al (2003) Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophys 14(8):841–848
Von Lukowicz T et al (2007) Human antibody against C domain of tenascin-C visualizes murine atherosclerotic plaques ex vivo. J Nucl Med 48(4):582–587
Vyavahare N et al (2000) Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol 157(3):885–893
Imanaka-Yoshida K, Matsumoto K-i (2018) Multiple roles of tenascins in homeostasis and pathophysiology of aorta. Ann Vasc Dis ra. 17–00118
Sato A et al (2006) Serum tenascin-C might be a novel predictor of left ventricular remodeling and prognosis after acute myocardial infarction. J Am Coll Cardiol 47(11):2319–2325
Schaff M et al (2011) Novel function of tenascin-C, a matrix protein relevant to atherosclerosis, in platelet recruitment and activation under flow. Arterioscler Thromb Vasc Biol 31:117–1241
Kajiwara K et al (2004) Tenascin-C is associated with coronary plaque instability in patients with acute coronary syndromes. Circ J 68(3):198–203
Cowan KN, Jones PL, Rabinovitch M (2000) Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Investig 105(1):21–34
Yildiz OP et al (2015) Relation between coronary artery calcium score and serum tenascin-C level in patients without known coronary artery disease. Acta Cardiol 70(6):633–639
Group BDW et al (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework, vol 69. Clinical pharmacology & therapeutics, pp 89–95. 3
Oldenhuis C et al (2008) Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer 44(7):946–953
Brase JC et al (2010) Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer 9(1):1–9
Radwanska A et al (2017) Counterbalancing anti-adhesive effects of Tenascin-C through fibronectin expression in endothelial cells. Sci Rep 7(1):1–14
Saupe F et al (2013) Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep 5(2):482–492
Ando K et al (2011) Tenascin C may regulate the recruitment of smooth muscle cells during coronary artery development. Differentiation 81(5):299–306
Ludmer PL et al (1986) Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315(17):1046–1051
Zagzag D et al (2002) Tenascin-C promotes microvascular cell migration and phosphorylation of focal adhesion kinase. Cancer Res 62(9):2660–2668
Chung C, Murphy-Ullrich J, Erickson H (1996) Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell 7(6):883–892
Giblin S, Midwood K (2015) Tenascin-C: form versus function. Cell Adh Migr 9 (1–2):48–82
Acknowledgements
The authors thank Dr Ali Sharifi-Zarchi for his kind assistance in data analysis. This work was supported by a research grant to Dr Malakootian awarded by the Research Deputyship of Rajaie Cardiovascular Medical and Research Center (94111).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study was approved by the ethics committee of Rajaie Cardiovascular Medical and Research Center “Mahshid Malakootian” (ethics code: RHC.ac.ir.REC.1396.61). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material

11033_2022_7760_MOESM1_ESM.png
The image depicts the TNC expression levels in the three subgroups of CAD patients. The expression level of Tenascin-C (TNC) is not statistically significant between single vessel, two vessels and three vessels CAD patients (p > 0.05)
Rights and permissions
About this article
Cite this article
Gholipour, A., Shakerian, F., Zahedmehr, A. et al. Tenascin-C as a noninvasive biomarker of coronary artery disease. Mol Biol Rep 49, 9267–9273 (2022). https://doi.org/10.1007/s11033-022-07760-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07760-z