Abstract
Background
Glioblastoma (GBM) is the most malignant and the fastest-progressing type of primary brain tumours. Temozolomide (TMZ) is a chemotherapeutic drug for the treatment of GBM. Extracellular vesicles (EVs) have been recently confirmed to have a substantial role in the GBM, and their contents released from GBM cells have been considered a target for treatment. The purpose of this study is to evaluate the impact of TMZ on heat shock proteins (HSPs) derived from EVs originated from GBM cell lines (U87-MG and LN229) and the significance of EVs in response to chemotherapy in GBM.
Methods and results
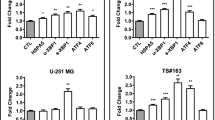
NTA, ELISA, and immunoblotting were used to characterization studies of EVs and results showed that U87-MG cells released many EVs compared to LN229 cells. The effect of TMZ treatments on HSPs expression levels were assessed with immunoblotting and was found to be led to increases in HSF-1, Hsp90, Hsp70, Hsp60 and Hsp27 expression in GBM cells and their EV contents, which these increases are related to therapeutic resistance. What is more, in Real-time PCR studies showing which signalling pathways might be associated with these increases, it was observed that TMZ triggered the expression of RAD51 and MDM2 genes in cells and EV contents. More strikingly, we discover a correlation between EV and parental cells in regard of mRNA and protein level in both cell lines as a result of TMZ treatment.
Conclusions
Our data suggest of EVs in the treatment of GBM may have potential biomarkers that can be used to investigate the treatment response.




Similar content being viewed by others
Data availability
The related detailed data from this study is available from the corresponding author.
Code availability
Not applicable.
Abbreviations
- ALIX:
-
Apoptosis-linked gene 2-interacting protein X
- EV:
-
Extracellular vesicle
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GBM:
-
Glioblastoma
- HSF:
-
Heat shock factor
- Hsp:
-
Heat shock protein
- IC50 :
-
The half-maximal inhibitory concentration
- MDM2:
-
Murine double-minute 2
- NTA,:
-
Nanoparticle tracking analysis
- RAD51,:
-
RAD51 recombinase
- TMZ,:
-
Temozolomide
- TSG101,:
-
Tumor susceptibility gene 101
References
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75(1):5–14. https://doi.org/10.1007/s11060-004-8093-6
Tabouret E, Chınot O, Metellus P et al (2012) Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 32(11):4655–4662
Alifieris C, Trafalis DT (2015) Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther 152:63–82. https://doi.org/10.1016/j.pharmthera.2015.05.005
Rappa F, Farina F, Zummo G et al (2012) HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res 32(12):5139–5150
Saibil H (2013) Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 14:630–642. https://doi.org/10.1038/nrm3658
Kim YE, Hipp MS, Bracher A et al (2013) Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82:323–355. https://doi.org/10.1146/annurev-biochem-060208-092442
Mertoglu E, Onay Ucar E (2021) Targeting heat shock protein 27 (HspB1) in glioblastoma cells with the combination of resveratrol and temozolomide. UHOD 31:221–229
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (1820) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 7:940–948. https://doi.org/10.1016/j.bbagen.2012.03.017
Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593. https://doi.org/10.1038/nri2567
Soung YH, Nguyen T, Cao H et al (2016) Emerging roles of exosomes in cancer invasion and metastasis. BMB Rep 49(1):18–25. https://doi.org/10.5483/BMBRep.2016.49.1.239
Zhang X, Yuan X, Shi H et al (2015) Exosomes in cancer: small particle, big player. J Hematol Oncol 8(1):83. https://doi.org/10.1186/s13045-015-0181-x
Van der Pol E, Böing AN, Harrison P et al (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64(3):676–705. https://doi.org/10.1124/pr.112.005983
Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: Extracellular organelles important in intercellular communication. J Proteomics 73(10):1907–1920. https://doi.org/10.1016/j.jprot.2010.06.006
Eguchi T, Sheta M, Fujii M, Calderwood SK (2022) Cancer extracellular vesicles, tumoroid models, and tumor microenvironment. Semin Cancer Biol 1044–579(22):00003–00007. https://doi.org/10.1016/j.semcancer
Schneider M, Winkler K, Kell R et al (2022) The chaperone protein grp78 promotes survival and migration of head and neck cancer after direct radiation exposure and extracellular vesicle-transfer. Front Oncol 12:842418. https://doi.org/10.3389/fonc.2022.842418
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Wessel D, Flügge UI (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138(1):141–143. https://doi.org/10.1016/0003-2697(84)90782-6
De Sousa AR, Penalva LO, Marcotte EM, Vogel C (2009) Global signatures of protein and mRNA expression levels. Mol Biosyst 5(12):1512–1526. https://doi.org/10.1039/b908315d
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232. https://doi.org/10.1038/nrg3185
Maier T, Güell M, Serrano L (2009) Correlation of mRNA and protein in complex biological samples. FEBS Lett 583(24):3966–3973. https://doi.org/10.1016/j.febslet.2009.10.036
Lin C-J, Lee C-C, Shih Y-L et al (2012) Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic Biol Med 52(2):377–391. https://doi.org/10.1016/j.freeradbiomed.2011.10.487
Lan F, Yang Y, Han J et al (2016) Sulforaphane reverses chemo-resistance to temozolomide in glioblastoma cells by NF-κB-dependent pathway downregulating MGMT expression. Int J Oncol 48(2):559–568. https://doi.org/10.3892/ijo.2015.3271
Lee SY (2016) Temozolomide resistance in glioblastoma multiforme. Genes Dis 3(3):198–210. https://doi.org/10.1016/j.gendis.2016.04.007
Akbarnejad Z, Eskandary H, Dini L et al (2017) Cytotoxicity of temozolomide on human glioblastoma cells is enhanced by the concomitant exposure to an extremely low-frequency electromagnetic field (100 Hz, 100 G). Biomed Pharmacother 92:254–264. https://doi.org/10.1016/j.biopha.2017.05.050
Yoshioka Y, Konishi Y, Kosaka N et al (2013) Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. https://doi.org/10.3402/jev.v2i0.20424
Simon T, Pinioti S, Schellenberger P et al (2018) Shedding of bevacizumab in tumour cells-derived extracellular vesicles as a new therapeutic escape mechanism in glioblastoma. Mol Can 17:132. https://doi.org/10.1186/s12943-018-0878-x
Tian Y, Li S, Song J et al (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35(7):2383–2390. https://doi.org/10.1016/j.biomaterials.2013.11.083
Chen TS, Arslan F, Yin Y et al (2011) Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med 9:47. https://doi.org/10.1186/1479-5876-9-47
Paolini A, Pasi F, Facoetti A et al (2011) Cell death forms and HSP70 expression in U87 cells after ionizing radiation and/or chemotherapy. Anticancer Res 31(11):3727–3731
Pasi F, Paolini A, Nano R et al (2014) Effects of single or combined treatments with radiation and chemotherapy on survival and danger signals expression in glioblastoma cell lines. Biomed Res Int 2014:453497. https://doi.org/10.1155/2014/453497
Castro GN, Cayado-Gutiérrez N, Zoppino FCM et al (2015) Effects of temozolomide (TMZ) on the expression and interaction of heat shock proteins (HSPs) and DNA repair proteins in human malignant glioma cells. Cell Stress Chaperones 20(2):253–265. https://doi.org/10.1007/s12192-014-0537-0
Sarto C, Binz P-A, Mocarelli P (2000) Heat shock proteins in human cancer. Electrophoresis 21(6):1218–1226. https://doi.org/10.1002/(SICI)1522-2683(20000401)21:6%3c1218:AID-ELPS1218%3e3.0.CO;2-H
Takashima M, Kuramitsu Y, Yokoyama Y et al (2003) Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics 3:2487–2493. https://doi.org/10.1002/pmic.200300621
André-Grégoire G, Bidère N, Gavard J (2018) Temozolomide affects extracellular vesicles released by glioblastoma cells. Biochimie 155:11–15. https://doi.org/10.1016/j.biochi.2018.02.007
Campanella C, Rappa F, Sciumè C et al (2015) Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 121(18):3230–3239. https://doi.org/10.1002/cncr.29499
Gobbo J, Marcion G, Cordonnier M et al (2016) Restoring anticancer immune response by targeting tumor-derived exosomes with a hsp70 peptide aptamer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv330
Wyciszkiewicz A, Kalinowska-Łyszczarz A, Nowakowski B et al (2019) Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Sci Rep 9:9817. https://doi.org/10.1038/s41598-019-46221-9
Eguchi T, Ono K, Kawata K, Okamoto K, Calderwood SK (2019) Regulatory roles of HSP90-rich extracellular vesicles. In: Asea A, Kaur P (eds) Heat shock protein 90 in human diseases and disorders heat shock proteins, 19. Springer, Cham, pp 3–17
Lv LH, Wan YL, Lin Y et al (2012) Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 287(19):15874–15885. https://doi.org/10.1074/jbc.M112.340588
Collis SJ (2001) Ribozyme minigene-mediated RAD51 down-regulation increases radiosensitivity of human prostate cancer cells. Nucleic Acids Res 29:1534–1538. https://doi.org/10.1093/nar/29.7.1534
Rapakko K, Heikkinen K, Karppinen S-M, Winqvist R (2006) Screening for RAD51 and BRCA2 BRC repeat mutations in breast and ovarian cancer families. Cancer Lett 236(1):142–147. https://doi.org/10.1016/j.canlet.2005.05.032
Zhang N, Wu X, Yang L et al (2012) FoxM1 Inhibition sensitizes resistant glioblastoma cells to temozolomide by downregulating the expression of DNA-repair gene Rad51. Clin Cancer Res 18:227–232. https://doi.org/10.1158/1078-0432.CCR-12-0039
Li Q, Ru Y, Lyu W et al (2019) RAD51 promotes proliferation and migration of glioblastoma cells and decreases sensitivity of cells to temozolomide. Chin J Cell Mol Immunol 35:817–822
Welsh JW, Ellsworth RK, Kumar R et al (2009) Rad51 Protein expression and survival in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys 74(4):1251. https://doi.org/10.1016/j.ijrobp.2009.03.018
Murakami N, Kühnel A, Schmid TE et al (2015) Role of membrane Hsp70 in radiation sensitivity of tumor cells. Radiat Oncol 10:149. https://doi.org/10.1186/s13014-015-0461-1
McLaughlin M, Barker HE, Khan AA et al (2017) HSP90 inhibition sensitizes head and neck cancer to platin-based chemoradiotherapy by modulation of the DNA damage response resulting in chromosomal fragmentation. BMC Cancer 17(1):86. https://doi.org/10.1186/s12885-017-3084-0
Dubrez L, Causse S, Borges Bonan N et al (2020) Heat-shock proteins: chaperoning DNA repair. Oncogene 39(3):516–529. https://doi.org/10.1038/s41388-019-1016-y
Ko JL, Cheng YW, Chang SL et al (2000) MDM2 mRNA expression is a favorable prognostic factor in non-small-cell lung cancer. Int J Cancer 89(3):265–270. https://doi.org/10.1002/1097-0215(20000520)89:3%3c265::AID-IJC9%3e3.0.CO;2-N
Vaughan C, Mohanraj L, Singh S et al (2011) Human oncoprotein MDM2 Up-regulates expression of NF- B2 precursor p100 conferring a survival advantage to lung cells. Genes Cancer 2(10):943–955. https://doi.org/10.1177/1947601911436008
Jones SN, Hancock AR, Vogel H et al (1998) Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci USA 95(26):15608–15612. https://doi.org/10.1073/pnas.95.26.15608
Suzuki A, Toi M, Yamamoto Y et al (1998) Role of MDM2 overexpression in doxorubicin resistance of breast carcinoma. Jpn J Cancer Res 89(2):221–227. https://doi.org/10.1111/j.1349-7006.1998.tb00552.x
Hayashi S, Ozaki T, Yoshida K et al (2006) p73 and MDM2 confer the resistance of epidermoid carcinoma to cisplatin by blocking p53. Biochem Biophys Res Commun 347(1):60–66. https://doi.org/10.1016/j.bbrc.2006.06.095
Sato A, Sunayama J, Matsuda K et al (2011) MEK-ERK signaling dictates dna-repair gene mgmt expression and temozolomide resistance of stem-like glioblastoma cells via the mdm2-p53 axis. Stem Cells 29(12):1942–1951. https://doi.org/10.1002/stem.753
Tracz-Gaszewska Z, Klimczak M, Biecek P et al (2017) Molecular chaperones in the acquisition of cancer cell chemoresistance with mutated TP53 and MDM2 up-regulation. Oncotarget 8(47):82123–82143. https://doi.org/10.18632/oncotarget.18899
Shao H, Chung J, Lee K et al (2015) Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun 6:6999. https://doi.org/10.1038/ncomms7999
Acknowledgements
We cordially thank to PhD candidate Aslıhan Şengelen and PhD candidate Yunus Aksüt, MSc Ayşen Güngör for their assistance to during experimental works.
Funding
This study was funded by Istanbul University Scientific Research Projects (BAP) Coordination Unit, Turkey (Grant Number: 33460) and The Scientific and Technological Research Council of Turkey (TUBITAK, Grant Number: 216S973).
Author information
Authors and Affiliations
Contributions
EK designed the study. EK, and ZA performed the study and EK analysed the results. EK wrote the manuscript. ZA and EOU drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare no competing interest.
Ethical approval
Not applicable.
Patient consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11033_2022_7714_MOESM1_ESM.tif
Supplementary file1 (TIF 800 kb). Supplementary material 1 Effects of TMZ on the viability of human glioma cancer cells U87-MG and LN229. TMZ treatments were associated with an increase in cell death in a dose- and time-dependent manner in (a) U87-MG and (b) LN229 cell lines
11033_2022_7714_MOESM2_ESM.xlsx
Supplementary file2 (XLSX 16 kb). Supplementary material 2 This is a list all genes analysed in this study and their fold change after the administration of 200 μM TMZ
11033_2022_7714_MOESM3_ESM.tif
Supplementary file3 (TIF 1858 kb). Supplementary material 3 Quality of EV-RNAs isolated were evaluated with Agilent Bioanalyzer
Rights and permissions
About this article
Cite this article
Kıyga, E., Adıgüzel, Z. & Önay Uçar, E. Temozolomide increases heat shock proteins in extracellular vesicles released from glioblastoma cells. Mol Biol Rep 49, 8701–8713 (2022). https://doi.org/10.1007/s11033-022-07714-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07714-5