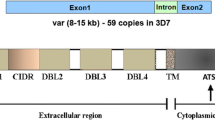
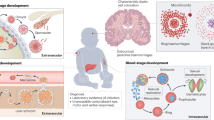
Abstract
Background
Cerebral malaria is often pronounced as a major life-threatening neurological complication of Plasmodium falciparum infection. The complex pathogenic landscape of the parasite and the associated neurological complications are still not elucidated properly. The growing concerns of drugresistant parasite strains along with the failure of anti-malarial drugs to subdue post-recovery neuro-cognitive dysfunctions in cerebral malaria patients have called for a demand to explore novel biomarkers and therapeutic avenues. Due course of the brain infection journey of the parasite, events such as sequestration of infected RBCs, cytoadherence, inflammation, endothelial activation, and blood–brain barrier disruption are considered critical.
Methods
In this review, we briefly summarize the diverse pathogenesis of the brain-invading parasite associated with loss of the blood-brain barrier integrity. In addition, we also discuss proteomics, transcriptomics, and bioinformatics strategies to identify an array of new biomarkers and drug candidates.
Conclusion
A proper understanding of the parasite biology and mechanism of barrier disruption coupled with emerging state-of-art therapeutic approaches could be helpful to tackle cerebral malaria.



Similar content being viewed by others
References
Idro R, Jenkins NE, Newton CRJ (2005) Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol 4:827–840. https://doi.org/10.1016/S1474-4422(05)70247-7
Idro R, Ndiritu M, Ogutu B et al (2007) Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA 297:2232–2240. https://doi.org/10.1001/jama.297.20.2232
Snow RW, Guerra CA, Noor AM et al (2005) The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214–217. https://doi.org/10.1038/nature03342
World Health Organization (2000) Severe falciparum malaria. Trans R Soc Trop Med Hyg 94:1–90. https://doi.org/10.1016/S0035-9203(00)90300-6
Hawkes M, Elphinstone RE, Conroy AL, Kain KC (2013) Contrasting pediatric and adult cerebral malaria. Virulence 4:543–555. https://doi.org/10.4161/viru.25949
Storm J, Jespersen JS, Seydel KB et al (2019) Cerebral malaria is associated with differential cytoadherence to brain endothelial cells. EMBO Mol Med 11(2):e9164
Storm J, Craig AG (2014) Pathogenesis of cerebral malaria—inflammation and cytoadherence. Front Cell Infect Microbiol 4:100
Nishanth G, Schlüter D (2019) Blood–brain barrier in cerebral malaria: pathogenesis and therapeutic intervention. Trend Parasitol 35:516–528. https://doi.org/10.1016/j.pt.2019.04.010
van der Heyde HC, Nolan J, Combes V et al (2006) A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trend Parasitol 22:503–508. https://doi.org/10.1016/j.pt.2006.09.002
Kadry H, Noorani B, Cucullo L (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barrier CNS 17:69. https://doi.org/10.1186/s12987-020-00230-3
Adams Y, Olsen RW, Bengtsson A et al (2021) Plasmodium falciparum erythrocyte membrane protein 1 variants induce cell swelling and disrupt the blood–brain barrier in cerebral malaria. J Exp Med 218:e20201266. https://doi.org/10.1084/jem.20201266
Tunon-Ortiz A, Lamb TJ (2019) Blood brain barrier disruption in cerebral malaria: beyond endothelial cell activation. PLoS Pathog 15:e1007786. https://doi.org/10.1371/journal.ppat.1007786
Ponsford MJ, Medana IM, Prapansilp P et al (2012) Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 205:663–671. https://doi.org/10.1093/infdis/jir812
Brown A, Turner L, Christoffersen S et al (2013) Molecular architecture of a complex between an adhesion protein from the malaria parasite and intracellular adhesion molecule 1. J Biol Chem 288:5992–6003. https://doi.org/10.1074/jbc.M112.416347
Hsieh F-L, Turner L, Bolla JR et al (2016) The structural basis for CD36 binding by the malaria parasite. Nat Commun 7:12837. https://doi.org/10.1038/ncomms12837
Lennartz F, Adams Y, Bengtsson A et al (2017) Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell Host Microbe 21:403–414. https://doi.org/10.1016/j.chom.2017.02.009
Marion A, Maria B, Maxwell B et al (2016) Interaction between endothelial protein c receptor and intercellular adhesion molecule 1 to mediate binding of Plasmodium falciparum-infected erythrocytes to endothelial cells. MBio 7:e00615-e616. https://doi.org/10.1128/mBio.00615-16
Faille D, Combes V, Mitchell AJ et al (2009) Platelet microparticles: a new player in malaria parasite cytoadherence to human brain endothelium. FASEB J 23:3449–3458. https://doi.org/10.1096/fj.09-135822
Wassmer SC, Combes V, Grau GER (2011) Platelets and microparticles in cerebral malaria: the unusual suspects. Drug Discov Today Dis Mech 8:e15–e23. https://doi.org/10.1016/j.ddmec.2011.11.004
Hochman SE, Madaline TF, Wassmer SC et al (2015) Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio 6(5):e01390-e1415
Gramaglia I, Velez J, Combes V et al (2017) Platelets activate a pathogenic response to blood-stage Plasmodium infection but not a protective immune response. Blood 129:1669–1679. https://doi.org/10.1182/blood-2016-08-733519
Sierro F, Grau GER (2019) The ins and outs of cerebral malaria pathogenesis: immunopathology, extracellular vesicles, immunometabolism, and trained immunity. Front Immunol 10:830
Burns AL, Dans MG, Balbin JM et al (2019) Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol Rev 43:223–238. https://doi.org/10.1093/femsre/fuz005
Mejia P, Treviño-Villarreal JH, Reynolds JS et al (2017) A single rapamycin dose protects against late-stage experimental cerebral malaria via modulation of host immunity, endothelial activation and parasite sequestration. Malar J 16:455. https://doi.org/10.1186/s12936-017-2092-5
Cariaco Y, Lima WR, Sousa R et al (2018) Ethanolic extract of the fungus Trichoderma stromaticum decreases inflammation and ameliorates experimental cerebral malaria in C57BL/6 mice. Sci Rep 8:1547. https://doi.org/10.1038/s41598-018-19840-x
Saiwaew S, Sritabal J, Piaraksa N et al (2017) Effects of sevuparin on rosette formation and cytoadherence of Plasmodium falciparum infected erythrocytes. PLoS ONE 12:e0172718. https://doi.org/10.1371/journal.pone.0172718
Leitgeb AM, Charunwatthana P, Rueangveerayut R et al (2017) Inhibition of merozoite invasion and transient de-sequestration by sevuparin in humans with Plasmodium falciparum malaria. PLoS ONE 12:e0188754. https://doi.org/10.1371/journal.pone.0188754
Taoufiq Z, Pino P, N’dilimabaka N et al (2011) Atorvastatin prevents Plasmodium falciparum cytoadherence and endothelial damage. Malar J 10:52. https://doi.org/10.1186/1475-2875-10-52
Franke-Fayard B, Janse CJ, Cunha-Rodrigues M et al (2005) Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci USA 102:11468–11473. https://doi.org/10.1073/pnas.0503386102
Bernabeu M, Smith JD (2017) EPCR and malaria severity: the center of a perfect storm. Trend Parasitol 33:295–308. https://doi.org/10.1016/j.pt.2016.11.004
Kessler A, Dankwa S, Bernabeu M et al (2017) Linking EPCR-binding PfEMP1 to brain swelling in pediatric cerebral malaria. Cell Host Microbe 22:601-614.e5. https://doi.org/10.1016/j.chom.2017.09.009
Mandala WL, Msefula CL, Gondwe EN et al (2017) Cytokine profiles in malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol 24:e00533-e616. https://doi.org/10.1128/CVI.00533-16
Dunst J, Kamena F, Matuschewski K (2017) Cytokines and chemokines in cerebral malaria pathogenesis. Front Cell Infect Microbiol 7:324
Sorensen EW, Lian J, Ozga AJ et al (2018) CXCL10 stabilizes T cell–brain endothelial cell adhesion leading to the induction of cerebral malaria. JCI Insight. https://doi.org/10.1172/jci.insight.98911
Wilson NO, Solomon W, Anderson L et al (2013) Pharmacologic inhibition of CXCL10 in combination with anti-malarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS ONE 8:e60898. https://doi.org/10.1371/journal.pone.0060898
Gun SY, Claser C, Teo TH et al (2018) Interferon regulatory factor 1 is essential for pathogenic CD8+ T cell migration and retention in the brain during experimental cerebral malaria. Cell Microbiol 20:e12819. https://doi.org/10.1111/cmi.12819
Schmidt KE, Kuepper JM, Schumak B et al (2018) Doxycycline inhibits experimental cerebral malaria by reducing inflammatory immune reactions and tissue-degrading mediators. PLoS ONE 13:e0192717. https://doi.org/10.1371/journal.pone.0192717
Crowley VM, Ayi K, Lu Z et al (2017) Synthetic oleanane triterpenoids enhance blood brain barrier integrity and improve survival in experimental cerebral malaria. Malar J 16:463. https://doi.org/10.1186/s12936-017-2109-0
Engwerda CR, Mynott TL, Sawhney S et al (2002) Locally up-regulated lymphotoxin α, not systemic tumor necrosis factor α, is the principle mediator of murine cerebral malaria. J Exp Med 195:1371–1377. https://doi.org/10.1084/jem.20020128
Lell B, Köhler C, Wamola B et al (2010) Pentoxifylline as an adjunct therapy in children with cerebral malaria. Malar J 9:368. https://doi.org/10.1186/1475-2875-9-368
Schmid U, Stenzel W, Koschel J et al (2017) The deubiquitinating enzyme cylindromatosis dampens CD8+ T cell responses and is a critical factor for experimental cerebral malaria and blood–brain barrier damage. Front Immunol 1(8):27
Swanson PA II, Hart GT, Russo MV et al (2016) CD8+ T cells induce fatal brainstem pathology during cerebral malaria via luminal antigen-specific engagement of brain vasculature. PLoS Pathog 12:e1006022. https://doi.org/10.1371/journal.ppat.1006022
Niewold P, Cohen A, van Vreden C et al (2018) Experimental severe malaria is resolved by targeting newly-identified monocyte subsets using immune-modifying particles combined with artesunate. Commun Biol 1:227. https://doi.org/10.1038/s42003-018-0216-2
Schumak B, Klocke K, Kuepper JM et al (2015) Specific depletion of Ly6Chi inflammatory monocytes prevents immunopathology in experimental cerebral malaria. PLoS ONE 10:e0124080. https://doi.org/10.1371/journal.pone.0124080
Besnard A-G, Guabiraba R, Niedbala W et al (2015) IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid Cells, M2 macrophages and regulatory T cells. PLoS Pathog 11:e1004607. https://doi.org/10.1371/journal.ppat.1004607
Pankoui Mfonkeu JB, Gouado I, Fotso Kuaté H et al (2010) Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS ONE 5:e13415. https://doi.org/10.1371/journal.pone.0013415
Piguet PF, Kan CD, Vesin C (2002) Thrombocytopenia in an animal model of malaria is associated with an increased caspase-mediated death of thrombocytes. Apoptosis 7:91–98. https://doi.org/10.1023/A:1014341611412
Combes V, Taylor TE, Juhan-Vague I et al (2004) Circulating endothelial microparticles in malawian children with severe falciparum malaria complicated with coma. JAMA 291:2542–2544. https://doi.org/10.1001/jama.291.21.2542-b
Dandewad V, Vindu A, Joseph J, Seshadri V (2019) Import of human miRNA-RISC complex into Plasmodium falciparum and regulation of the parasite gene expression. J Biosci 44:50. https://doi.org/10.1007/s12038-019-9870-x
Ye W, Chew M, Hou J et al (2018) Microvesicles from malaria-infected red blood cells activate natural killer cells via MDA5 pathway. PLoS Pathog 14:e1007298. https://doi.org/10.1371/journal.ppat.1007298
Wheway J, Latham SL, Combes V, Grau GER (2014) Endothelial microparticles interact with and support the proliferation of T cells. J Immunol 193:3378–3387. https://doi.org/10.4049/jimmunol.1303431
Sisquella X, Ofir-Birin Y, Pimentel MA et al (2017) Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nat Commun 8:1985. https://doi.org/10.1038/s41467-017-02083-1
El-Assaad F, Wheway J, Hunt NH et al (2014) Production, fate and pathogenicity of plasma microparticles in murine cerebral malaria. PLoS Pathog 10:e1003839. https://doi.org/10.1371/journal.ppat.1003839
Penet M-F, Abou-Hamdan M, Coltel N et al (2008) Protection against cerebral malaria by the low-molecular-weight thiol pantethine. Proc Natl Acad Sci USA 105(44):1321–1326. https://doi.org/10.1073/pnas.0706867105
Nantakomol D, Dondorp AM, Krudsood S et al (2011) Circulating red cell-derived microparticles in human malaria. J Infect Dis 203:700–706. https://doi.org/10.1093/infdis/jiq104
Combes V, Coltel N, Alibert M et al (2005) ABCA1 gene deletion protects against cerebral malaria: potential pathogenic role of microparticles in neuropathology. Am J Pathol 166:295–302. https://doi.org/10.1016/S0002-9440(10)62253-5
Cohen A, Zinger A, Tiberti N et al (2018) Differential plasma microvesicle and brain profiles of microRNA in experimental cerebral malaria. Malar J 17:192. https://doi.org/10.1186/s12936-018-2330-5
Ketprasit N, Cheng IS, Deutsch F et al (2020) The characterization of extracellular vesicles-derived microRNAs in Thai malaria patients. Malar J 19:285. https://doi.org/10.1186/s12936-020-03360-z
Barker KR, Lu Z, Kim H et al (2017) miR-155 modifies inflammation, endothelial activation and blood–brain barrier dysfunction in cerebral malaria. Mol Med 23:24–33. https://doi.org/10.2119/molmed.2016.00139
Ghosh S, Sengupta A, Sharma S, Sonawat HM (2012) Metabolic fingerprints of serum, brain, and liver are distinct for mice with cerebral and noncerebral malaria: a 1H NMR spectroscopy-based metabonomic study. J Proteome Res 11:4992–5004. https://doi.org/10.1021/pr300562m
Hunt NH, Manduci N, Thumwood CM (1993) Amelioration of murine cerebral malaria by dietary restriction. Parasitology 107:471–476. https://doi.org/10.1017/S0031182000068049
Mejia P, Treviño-Villarreal JH, Hine C et al (2015) Dietary restriction protects against experimental cerebral malaria via leptin modulation and T-cell mTORC1 suppression. Nat Commun 6:6050. https://doi.org/10.1038/ncomms7050
Gordon EB, Hart GT, Tran TM et al (2015) Targeting glutamine metabolism rescues mice from late-stage cerebral malaria. Proc Natl Acad Sci USA 112:13075–13080. https://doi.org/10.1073/pnas.1516544112
Wang A, Huen SC, Luan HH et al (2018) Glucose metabolism mediates disease tolerance in cerebral malaria. Proc Natl Acad Sci USA 115:11042–11047. https://doi.org/10.1073/pnas.1806376115
Zuzarte-Luís V, Mello-Vieira J, Marreiros IM et al (2017) Dietary alterations modulate susceptibility to Plasmodium infection. Nat Microbiol 2:1600–1607. https://doi.org/10.1038/s41564-017-0025-2
Holmberg D, Franzén-Röhl E, Idro R et al (2017) Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malar J 16:303. https://doi.org/10.1186/s12936-017-1954-1
Guillemin GJ (2012) Quinolinic acid, the inescapable neurotoxin. FEBS J 279:1356–1365. https://doi.org/10.1111/j.1742-4658.2012.08485.x
Bosco MC, Rapisarda A, Massazza S et al (2000) The tryptophan catabolite picolinic acid selectively induces the chemokines macrophage inflammatory protein-1α and -1β in macrophages. J Immunol 164:3283–3291. https://doi.org/10.4049/jimmunol.164.6.3283
Mathema VB, Na-Bangchang K (2015) A brief review on biomarkers and proteomic approach for malaria research. Asian Pac J Trop Med 8:253–262. https://doi.org/10.1016/S1995-7645(14)60327-8
Swearingen KE, Lindner SE (2018) Plasmodium parasites viewed through proteomics. Trend Parasitol 34:945–960. https://doi.org/10.1016/j.pt.2018.08.003
Galassie AC, Link AJ (2015) Proteomic contributions to our understanding of vaccine and immune responses. Proteomics Clin Appl 9(11–12):972–989. https://doi.org/10.1002/prca.201500054
Nilsson Bark SK, Ahmad R, Dantzler K et al (2018) Quantitative proteomic profiling reveals novel Plasmodium falciparum surface antigens and possible vaccine candidates. Mol Cell Proteomics 17:43–60. https://doi.org/10.1074/mcp.RA117.000076
Bachmann J, Burté F, Pramana S et al (2014) Affinity proteomics reveals elevated muscle proteins in plasma of children with cerebral malaria. PLoS Pathog 10:e1004038. https://doi.org/10.1371/journal.ppat.1004038
Gitau EN, Kokwaro GO, Karanja H et al (2013) Plasma and cerebrospinal proteomes from children with cerebral malaria differ from those of children with other encephalopathies. J Infect Dis 208:1494–1503. https://doi.org/10.1093/infdis/jit334
Zhang Y, Huang C, Kim S et al (2015) Multiple stiffening effects of nanoscale knobs on human red blood cells infected with Plasmodium falciparum malaria parasite. Proc Natl Acad Sci USA 112:6068–6073. https://doi.org/10.1073/pnas.1505584112
Florens L, Liu X, Wang Y et al (2004) Proteomics approach reveals novel proteins on the surface of malaria-infected erythrocytes. Mol Biochem Parasitol 135:1–11. https://doi.org/10.1016/j.molbiopara.2003.12.007
Bertin GI, Sabbagh A, Argy N et al (2016) Proteomic analysis of Plasmodium falciparum parasites from patients with cerebral and uncomplicated malaria. Sci Rep 6:26773. https://doi.org/10.1038/srep26773
Kumar M, Varun CN, Dey G et al (2018) Identification of host-response in cerebral malaria patients using quantitative proteomic analysis. Proteomics Clin Appl 12(4):1600187
Abdi AI, Yu L, Goulding D et al (2017) Proteomic analysis of extracellular vesicles from a Plasmodium falciparum Kenyan clinical isolate defines a core parasite secretome. Wellcome Open Res 2:50
Mantel P-Y, Hoang AN, Goldowitz I et al (2013) Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13:521–534. https://doi.org/10.1016/j.chom.2013.04.009
Boldt ABW, van Tong H, Grobusch MP et al (2019) The blood transcriptome of childhood malaria. EBioMedicine 40:614–625. https://doi.org/10.1016/j.ebiom.2018.12.055
Beri D, Ramdani G, Balan B et al (2019) Insights into physiological roles of unique metabolites released from Plasmodium-infected RBCs and their potential as clinical biomarkers for malaria. Sci Rep 9:2875. https://doi.org/10.1038/s41598-018-37816-9
Moussa E, Huang H, Ahras M et al (2018) Proteomic profiling of the brain of mice with experimental cerebral malaria. J Proteomics 180:61–69. https://doi.org/10.1016/j.jprot.2017.06.002
Moussa EM, Huang H, Thézénas ML et al (2018) Proteomic profiling of the plasma of Gambian children with cerebral malaria. Malar J 17:337. https://doi.org/10.1186/s12936-018-2487-y
Sampaio NG, Cheng L, Eriksson EM (2017) The role of extracellular vesicles in malaria biology and pathogenesis. Malar J 16:245. https://doi.org/10.1186/s12936-017-1891-z
Thiam A, Sanka M, Ndiaye Diallo R et al (2019) Gene expression profiling in blood from cerebral malaria patients and mild malaria patients living in senegal. BMC Med Genomic 12:148. https://doi.org/10.1186/s12920-019-0599-z
Almelli T, Nuel G, Bischoff E et al (2014) Differences in gene transcriptomic pattern of Plasmodium falciparum in children with cerebral malaria and asymptomatic carriers. PLoS ONE 9:e114401. https://doi.org/10.1371/journal.pone.0114401
Nambou K, Nie X, Tong Y, Anakpa M (2021) Weighted gene co-expression network analysis and drug–gene interaction bioinformatics uncover key genes associated with various presentations of malaria infection in african children and major drug candidates. Infect Genet Evol 89:104723. https://doi.org/10.1016/j.meegid.2021.104723
Nallandhighal S, Park GS, Ho Y-Y et al (2019) Whole-blood transcriptional signatures composed of erythropoietic and NRF2-regulated genes differ between cerebral malaria and severe malarial anemia. J Infect Dis 219:154–164. https://doi.org/10.1093/infdis/jiy468
Oakley MS, Anantharaman V, Venancio TM et al (2011) Molecular correlates of experimental cerebral malaria detectable in whole blood. Infect Immun 79:1244–1253. https://doi.org/10.1128/IAI.00964-10
Malaria Genomic Epidemiology Network (2019) Insights into malaria susceptibility using genome-wide data on 17,000 individuals from Africa. Asia Ocean Nat Commun 10:5732. https://doi.org/10.1038/s41467-019-13480-z
Damena D, Denis A, Golassa L, Chimusa ER (2019) Genome-wide association studies of severe P. falciparum malaria susceptibility progress, pitfalls and prospects. BMC Med Genomics 12(1):120
Tan QW, Mutwil M (2020) Malaria.tools—comparative genomic and transcriptomic database for Plasmodium species. Nucleic Acid Res 48:D768–D775. https://doi.org/10.1093/nar/gkz662
Aurrecoechea C, Brestelli J, Brunk BP et al (2009) PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acid Res 37:D539–D543. https://doi.org/10.1093/nar/gkn814
Rout S, Mahapatra RK (2019) In silico analysis of Plasmodium falciparum CDPK5 protein through molecular modeling, docking and dynamics. J Theor Biol 461:254–267. https://doi.org/10.1016/j.jtbi.2018.10.045
Rout S, Mahapatra RK (2019) In silico study of M18 aspartyl amino peptidase (M18AAP) of Plasmodium vivax as an antimalarial drug target. Bioorg Med Chem 27:2553–2571. https://doi.org/10.1016/j.bmc.2019.03.039
Rout S, Warhurst DC, Suar M, Mahapatra RK (2015) In silico comparative genomics analysis of Plasmodium falciparum for the identification of putative essential genes and therapeutic candidates. J Microbiol Method 109:1–8. https://doi.org/10.1016/j.mimet.2014.11.016
Liu X, Wang Y, Liang J et al (2018) In-depth comparative analysis of malaria parasite genomes reveals protein-coding genes linked to human disease in Plasmodium falciparum genome. BMC Genomics 19:312. https://doi.org/10.1186/s12864-018-4654-5
Liu X, Wu Y, Zhao Y et al (2021) Identification of Plasmodium falciparum-specific protein PIESP2 as a novel virulence factor related to cerebral malaria. Int J Biol Macromol 177:535–547. https://doi.org/10.1016/j.ijbiomac.2021.02.145
Talundzic E, Ravishankar S, Kelley J et al (2018) Next-generation sequencing and bioinformatics protocol for malaria drug resistance marker surveillance. Antimicrob Agent Chemother 62:e02474-e2517. https://doi.org/10.1128/AAC.02474-17
Funding
The work has not been sponsored by any external funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Ethical approval
The review article is complied with ethical standard.
Research involving human and/or animal participants
The current report contains no human or animal studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panda, C., Mahapatra, R.K. An update on cerebral malaria for therapeutic intervention. Mol Biol Rep 49, 10579–10591 (2022). https://doi.org/10.1007/s11033-022-07625-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07625-5