Abstract
Background
Congenital Anomalies of the Kidney and the Urinary Tract (CAKUT) are defined as a heterogeneous group of anomalies that resulted from defects in kidney and urinary tract embryogenesis. CAKUT have a complex etiology. Genetic, epigenetic and environmental factors have been investigated in this context. Angiotensin II is a potent vasoconstrictor and exerts an important role in kidney embryogenesis. The angiotensin-converting enzyme (ACE) converts Angiotensin I into Angiotensin II (Ang II) and ACE gene has insertion/deletion (I/D) polymorphisms that have been evaluated in several nephropathies. This study aimed to evaluate whether the I/D polymorphisms of ACE gene and the circulating levels of Ang II are associated with any CAKUT phenotype or CAKUT in general.
Methods and results
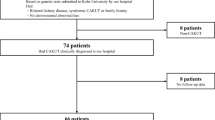
Our study was performed with 225 pediatric patients diagnosed with CAKUT and 210 age-and-sex matched healthy controls. ACE I/D alleles were analysed by real-time polymerase chain reaction (RT-PCR). The distribution of ACE I/D polymorphisms were compared between CAKUT patients and healthy controls, as well between ureteropelvic junction obstruction (UPJO), vesicoureteral reflux (VUR), multicystic dysplastic kidney (MCDK) phenotypes and control group. No statistical association was detected between ACE I/D polymorphism and CAKUT and UPJO, VUR, and MCDK phenotypes. In a subset of 80 CAKUT patients and 80 controls, plasma levels of Ang II were measured. No significant differences were found between CAKUT patients and controls, even in regard to comparisons of UPJO, VUR and MCDK with control group.
Conclusion
Although CAKUT is a complex disease and the ACE gene may exert a role in kidney embryogenesis, CAKUT was not associated with any ACE I/D polymorphisms nor with differences in plasma levels of Ang II in this Brazilian pediatric population.

Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Schütz S, Le Moullec JM, Corvol P, Gasc JM (1996) Early expression of all the components of the renin-angiotensin-system in human development. Am J Pathol 149:2067–2079
Heikkila J, Holmberg C, Kyllonen L, Rintala R, Taskinen S (2011) Long term risk of end stage renal disease in patients with posterior urethral valves. J Urol 186:2392–2396
Negrisolo S, Benetti E, Centi S, Vella MD, Ghirardo G, Zanon G et al (2011) PAX2 gene mutations in pediatric and young adult transplant recipients: kidney and urinary tract malformations without ocular anomalies. Clin Genet 80(6):581–585
Finer G, Shalev H, Landau D (2006) Genetic kidney diseases in the pediatric population of southern Israel. Pediatr Nephrol 21(7):910–916
Fletcher J, McDonald S, Alexander SI, Australian and New Zealand Pediatric Nephrology Association (ANZPNA) (2013) Prevalence of genetic renal disease in children. Pediatr Nephrol 28(2):251–256
Melo BF, Aguiar MB, Bouzada MC, Aguiar RL, Pereira AK, Paixao GM, Linhares MC, Valerio FC, Simões e Silva AC, Oliveira EA (2012) Early risk factors for neonatal mortality in CAKUT: analysis of 524 affected newborns. Pediatr Nephrol 27(6):965–972
Quirino IG, Diniz JS, Bouzada MC, Pereira AK, Lopes TJ, Paixão GM et al (2012) Clinical course of 822 children with prenatally detected nephrouropathies. Clin J Am Soc Nephrol 7(3):444–451. https://doi.org/10.2215/CJN.03400411
Miranda DM, Santos-Junior ACS, Dos Reis GS, Freitas IS, Carvalho TGR, de Marco LAC et al (2014) PAX2 polymorphisms and congenital abnormalities of the kidney and urinary tract in a Brazilian pediatric population: evidence for a role in vesicoureteral reflux. Mol Diagn Ther 18:451–457
Miranda DM, Santos-Junior ACS, Sarubi HC, Bastos-Rodrigues L, Rosa DV, Freitas IS et al (2014) Association of angiotensin type 2 receptor gene polymorphisms with ureteropelvic junction obstruction in Brazilian patients. Nephrology 19:714–720
Rahimi Z (2012) ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathol 1:143–151
Sayed-Tabatabaei FA, Oostra BA, Isaacs A, van Duijn CM, Witteman JCM (2006) ACE polymorphisms. Circul Res 98:1123–1133
Yu Z-Y, Chen L-S, Zhang L-C, Zhou T-B (2012) Meta-analysis of the relationship between ACE I/D gene polymorphism and end-stage renal disease in patients with diabetic nephropathy: ACE and ESRD in DN patients. Nephrology 17:480–487
Santos-Junior ACS, Miranda DM, Simões e Silva AC (2014) Congenital anomalies of the kidney and urinary tract: an embryogenetic review. Birth Defects Res C Embryo Today 102:374–381
Zhang S-L (2004) Angiotensin II increases pax-2 expression in fetal kidney cells via the AT2 receptor. J Am Soc Nephrol 15:1452–1465
Rabelo EAS, Oliveira EA, Silva JMP, Bouzada MCF, Sousa BC, Almeida MN et al (2005) Conservative management of multicystic dysplasic kidney: clinical course and ultrasound outcome. J Pediatr (Rio J) 81(5):400–404
Dias CS, Silva JMP, Pereira AK, Marino VS, Silva LA, Coelho AM et al (2013) Diagnostic accuracy of renal pelvic dilatation for detecting surgically managed ureteropelvic junction obstruction. J Urol 190:661–666
IBGE (2010) Censo 2010. Brazilian Institute of Geography and Statistics. Brazilian Institute of Geography and Statistics. https://censo2010.ibge.gov.br
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warad BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Nicolaou N, Renkema KY, Bongers EMHF, Giles RH, Knoers NVAM (2015) Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11:720–731
Simões e Silva AC, Lanza K, Palmeira VA, Costa LB, Flynn JT (2021) 2020 update on the renin-angiotensin-aldosterone system in pediatric kidney disease and its interactions with coronavirus. Pediatr Nephrol 36:1407–1426
Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86:1343–1346
Sequeira Lopez MLS, Gomez RA (2004) The role of angiotensin II in kidney embryogenesis and kidney abnormalities. Curr Opin Nephrol Hypertens 13:117–122
Guron G, Friberg P (2000) An intact renin—angiotensin system is a prerequisite for normal renal development. J Hypertens 18(2):123–137
Song R, Preston G, Yosypiv IV (2011) Angiotensin II stimulates in vitro branching morphogenesis of the isolated ureteric bud. Mech Dev 128:359–367
Yosypiv IV (2008) A new role for the renin-angiotensin system in the development of the ureteric bud and renal collecting system. Keio J Med 57:184–189
Song R, Spera M, Garrett C, El-Dahr SS, Yosypiv IV (2010) Angiotensin II AT2 receptor regulates ureteric bud morphogenesis. Am J Physiol Renal Physiol 298:F807-817
Hiraoka M, Taniguchi T, Nakai H, Kino M, Okada Y, Tanizawa A et al (2001) No evidence for AT2R gene derangement in human urinary tract anomalies. Kidney Int 59:1244–1249
Zhou T-B, Lin N, Liu Y-G, Qin Y-H, Shao M-B, Peng D-D (2012) Association of ACE I/D gene polymorphism with vesicoureteral reflux susceptibility in children: a meta-analysis. J Renin Angiotensin Aldosterone Syst 13:273–281
Kostadinova ES, Miteva LD, Stanilova SA (2017) ACE serum level and I/D gene polymorphism in children with obstructive uropathies and other congenital anomalies of the kidney and urinary tract. Nephrology 22:609–616
Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD (2003) Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA 100:177–182
Funding
This work was partially supported by Brazilian National Council of Research Development (CNPq—Grant # 302153/2019-5), Coordination of High Education Level Personnel (CAPES) and Foundation of Research of Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Contributions
PAP, TSCM and LMF wrote the first draft. ARB performed laboratorial and statistical analyses. ACSS and ARB conceptualized the study, made general supervision and revised the manuscript. ACSS submitted the final version of the manuscript, which is approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they no conflict of interest.
Ethical approval
This study was approved by the local Ethics Committee.
Consent to participate
All subjects involved in this study gave consent and assented to participate in this study.
Consent for publication
All subjects involved in this study gave consent and assented for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pousa, P.A., Mendonça, T.S.C., Fonseca, L.M. et al. Evaluation of insertion/deletion (I/D) polymorphisms of ACE gene and circulating levels of angiotensin II in congenital anomalies of the kidney and urinary tract. Mol Biol Rep 49, 4341–4347 (2022). https://doi.org/10.1007/s11033-022-07269-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07269-5