Abstract
Background and objectives
Obesity is known as a disease with a chronic low-grade state of inflammation and high levels of oxidative stress. Given the challenges and consequences caused by obesity, obesity therapy is an essential subject to address. For sustainable weight loss, gastric bypass surgery is the most successful and essential option.
Methods
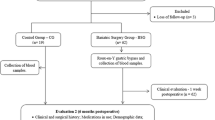
This prospective cohort study was performed on 35 patients aged (18–54) with morbid obesity (BMI: 42.06 kg/m2). Volunteers blood was taken, and peripheral blood mononuclear cells (PBMCs) were isolated, high mobility group box 1(HMGB1), nuclear factor erythroid2–related factor 2(Nrf2), Interleukin 6(IL-6), tumor necrosis factor-alpha (TNF-α), and biochemical factors were determined one day before and 4 months after surgery.
Results
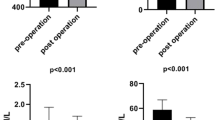
Four months following surgery, the BMI, hip and waist circumferences, and waist-to-hip ratio (WHR) all decreased significantly. The lipid profile and antioxidant power were dramatically enhanced after surgery. IL-6 and TNF-α expression in PBMC patients showed a significant decrease after surgery. HMGB1 and Nrf2 expression in PBMC of postoperative patients decreased compared to before surgery, and HMGB1, and Nrf2 protein levels also decreased after surgery.
Conclusion
Weight loss indicated the significant function of adipose tissue in the induction of oxidative stress and inflammatory factors. Gastric bypass reduced the inflammation conditions and improved the metabolic status and living situations in the patients with morbid obesity.



Similar content being viewed by others
References
Organization WH (2018) WHO Media Centre. Obesity and overweight: fact sheet (No. 311)
Samad F, Ruf W (2013) Inflammation, obesity, and thrombosis. Blood 122(20):3415–3422
Andersson U, Tracey KJ (2004) HMGB1 as a mediator of necrosis-induced inflammation and a therapeutic target in arthritis. Rheum Dis Clin 30(3):627–637
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418(6894):191–195
Magna M, Pisetsky DS (2016) The alarmin properties of DNA and DNA-associated nuclear proteins. Clin Ther 38(5):1029–1041
Wagner M (2014) A dangerous duo in adipose tissue: high-mobility group box 1 protein and macrophages. Yale J Biol Med 87(2):127
Gesta S, Tseng Y-H, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131(2):242–256
Park JS, Svetkauskaite D, He Q, Kim J-Y, Strassheim D, Ishizaka A et al (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279(9):7370–7377
Vanacore D, Messina G, Lama S, Bitti G, Ambrosio P, Tenore G et al (2018) Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: a pilot study. J Cell Physiol 233(12):9345–9353
Vincent HK, Taylor AG (2006) Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes 30(3):400–418
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y et al (2017) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Investig 114(12):1752–1761
More VR, Xu J, Shimpi PC, Belgrave C, Luyendyk JP, Yamamoto M et al (2013) Keap1 knockdown increases markers of metabolic syndrome after long-term high fat diet feeding. Free Radic Biol Med 61:85–94
de Haan JB (2011) Nrf2 activators as attractive therapeutics for diabetic nephropathy. Diabetes 60(11):2683–2684
Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S et al (2009) Reactive oxygen species enhance insulin sensitivity. Cell Metab 10(4):260–272
Taguchi K, Motohashi H, Yamamoto M (2011) Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 16(2):123–140
Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S et al (2009) Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol 620(1–3):138–144
Heneghan HM, Heinberg L, Windover A, Rogula T, Schauer PR (2012) Weighing the evidence for an association between obesity and suicide risk. Surg Obes Relat Dis 8(1):98–107
Wittgrove AC, Clark GW, Tremblay LJ (1994) Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg 4(4):353–357
Ingelfinger JR (2011) Bariatric surgery in adolescents. N Engl J Med 365(15):1365–1367
Farrell TM, Haggerty SP, Overby DW, Kohn GP, Richardson WS, Fanelli RD (2009) Clinical application of laparoscopic bariatric surgery: an evidence-based review. Surg Endosc 23(5):930–949
Valette M, Poitou C, Le Beyec J, Bouillot J-L, Clement K, Czernichow S (2012) Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS ONE 7(11):e48221
Zhou K, Wolski K, Malin SK, Aminian A, Schauer PR, Bhatt DL et al (2019) Impact of weight loss trajectory following randomization to bariatric surgery on long-term diabetes glycemic and cardiometabolic parameters. Endocr Pract 25(6):572–579
Organization WH (2016) World Health Organization obesity and overweight fact sheet
Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M et al (2018) The impact of obesity on the cardiovascular system. J Diabetes Res. https://doi.org/10.1155/2018/3407306
Pecht T, Haim Y, Bashan N, Shapiro H, Harman-Boehm I, Kirshtein B et al (2016) Circulating blood monocyte subclasses and lipid-laden adipose tissue macrophages in human obesity. PLoS ONE 11(7):e0159350
Choromańska B, Myśliwiec P, Łuba M, Wojskowicz P, Dadan J, Myśliwiec H et al (2020) A longitudinal study of the antioxidant barrier and oxidative stress in morbidly obese patients after bariatric surgery. Does the metabolic syndrome affect the redox homeostasis of obese people? J Clin Med 9(4):976
Rai MF, Sandell L (2011) Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr 21(2):131
Vincent HK, Bourguignon CM, Taylor AG (2010) Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J Hum Nutr Diet 23(1):20–29
Boccellino M, Camussi G, Giovane A, Ferro L, Calderaro V, Balestrieri C et al (2005) Platelet-activating factor regulates cadherin-catenin adhesion system expression and β-catenin phosphorylation during Kaposi’s sarcoma cell motility. Am J Pathol 166(5):1515–1522
Boccellino M, Giuberti G, Quagliuolo L, Marra M, D’Alessandro A, Fujita H et al (2004) Apoptosis induced by interferon-α and antagonized by EGF is regulated by caspase-3-mediated cleavage of gelsolin in human epidermoid cancer cells. J Cell Physiol 201(1):71–83
Karaouzene N, Merzouk H, Aribi M, Merzouk S, Berrouiguet AY, Tessier C et al (2011) Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: a comparison of older with young men. Nutr Metab Cardiovasc Dis 21(10):792–799
Kuo F-C, Huang Y-H, Lin F-H, Hung Y-J, Hsieh C-H, Lu C-H et al (2017) Circulating soluble IL-6 receptor concentration and visceral adipocyte size are related to insulin resistance in Taiwanese adults with morbid obesity. Metab Syndr Relat Disord 15(4):187–193
Pourrajab F, Sharifi M, Hekmatimoghaddam S, Khanaghaei M, Rahaie M (2016) Elevated levels of miR-499 protect ischemic myocardium against uric acid in patients undergoing off-pump CABG. Cor Vasa 58(6):e600–e608
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38(12):1103–1111
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ (2009) HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367–388
Lee EB (2011) Obesity, leptin, and Alzheimer’s disease. Ann N Y Acad Sci 1243:15
Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL et al (2008) NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther 325(2):655–664
Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M et al (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106(21):8665–8670
Vilarrasa N, Vendrell J, Sánchez-Santos R, Broch M, Megia A, Masdevall C et al (2007) Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-α receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol 67(5):679–686
Arrigo T, Chirico V, Salpietro V, Munafo C, Ferrau V, Gitto E et al (2013) High-mobility group protein B1: a new biomarker of metabolic syndrome in obese children. Eur J Endocrinol 168(4):631–638
Giacobbe A, Grasso R, Imbesi G, Salpietro C, Grasso L, Lagana A et al (2015) High mobility group protein B1: a new biomarker of obesity in pregnant women? Gynecol Endocrinol 31(2):113–115
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig 112(12):1796–1808
Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K (2016) Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev 17(11):1067–1079
Viana EC, Araujo-Dasilio KL, Miguel GPS, Bressan J, Lemos EM, Moyses MR et al (2013) Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-α. Prospective clinical trial. Obes Surg 23(8):1252–1261
Nativel B, Marimoutou M, Thon-Hon VG, Gunasekaran MK, Andries J, Stanislas G et al (2013) Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: contribution to chronic inflammation in fat tissue. PLoS ONE 8(9):e76039
Jin C, Henao-Mejia J, Flavell RA (2013) Innate immune receptors: key regulators of metabolic disease progression. Cell Metab 17(6):873–882
Montes V, Subramanian S, Goodspeed L, Wang S, Omer M, Bobik A et al (2015) Anti-HMGB1 antibody reduces weight gain in mice fed a high-fat diet. Nutr Diabetes 5(6):e161
Borbély YM, Osterwalder A, Kröll D, Nett PC, Inglin RA (2017) Diarrhea after bariatric procedures: diagnosis and therapy. World J Gastroenterol 23(26):4689
Guman M, van Olst N, Yaman Z, Voermans R, de Brauw L, Nieuwdorp M et al (2021) Pancreatic exocrine insufficiency after bariatric surgery. Surg Obes Relat Dis. https://doi.org/10.1016/j.soard.2021.12.017
Acknowledgements
This report was taken from a PhD thesis at Hamadan University of Medical Sciences, Iran. The research was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences, Iran (No. 9811158686).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no contradiction with respect to the research, authorship, and/or publication of this article.
Ethical approval
The study was approved by the Ethics Committee of the Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.910).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khanaghaei, M., Ziamajidi, N., Poorolajal, J. et al. The influence of gastric bypass surgery on the concentration of high mobility group box 1, nuclear factor erythroid 2–related factor 2 and the genes expression of high mobility group box 1, nuclear factor erythroid2–related factor 2, interleukin 6, and tumor necrosis factor-alpha in the peripheral blood mononuclear cells of patients with morbid obesity. Mol Biol Rep 49, 3745–3755 (2022). https://doi.org/10.1007/s11033-022-07214-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07214-6