Abstract
Background
Coronary artery disease (CAD) is considered to be one of the most pivotal causes of death in the world. Over the past two decades, significant changes occurred in the diagnosis, prognosis, and treatment of CAD, which has helped reduce mortality rates. microRNAs (miRs) are a class of more than 5000 non-encoding RNA molecules (21–25 nucleotides across the length) that regulate complex biological processes. Today, miRNAs are used to study cardiovascular diseases. In the present study, the expression of miR-146a،miR-27, miR-149, and miR-34a in plasma suffering from CAD and the control group were investigated.
Methods and results
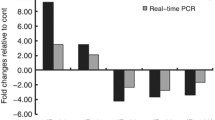
The present research was performed on 30 men with CAD and 30 healthy men as controls. The expression levels of miR-146a, miR-27a, miR-149, and miR-34a in the plasma of patients with CAD and the control group were measured using real-time PCR. Also, the correlation between the expression of circulating miRs levels and biochemical LDL-C, HDL-C, BMI, and total cholesterol was evaluated. The expression of miR-27a in the plasma of the CAD group was higher than in the control group (p = 0.020). The expression of miR-146a was downregulated in CAD patients compared to normal subjects (p = 0. 026). However, the expression of miR-34a, miR-149 in the plasma of CAD patients was not significantly different with the control group. In addition to, a direct correlation was found between the expression of miR-146a and HDL-c, the expression of miR-27a and LDL-C and the expression of miR-34a and total cholesterol. Also, the negative correlation between expressions of miR-149 with BMI was reported.
Conclusion
The obtained results demonstrated that miRs were closely related to biochemical factors and it points out the fact that miRNAs can be applied as a potential strategy for diagnosis and treatment of CAD.



Similar content being viewed by others
References
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473(7347):317–325
Sanz J, Moreno PR, Fuster V (2013) The year in atherothrombosis. J Am Coll Cardiol 62(13):1131–1143
Fichtlscherer S et al (2010) Circulating microRNAs in patients with coronary artery disease. Circ Res 107(5):677–684
Mitchell PS et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci 105(30):10513–10518
Selbach M et al (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455(7209):58–63
Hu J et al (2019) RBFox2-miR-34a-Jph2 axis contributes to cardiac decompensation during heart failure. Proc Natl Acad Sci 116(13):6172–6180
Shen L et al (2019) Downregulation of miR-146a contributes to cardiac dysfunction induced by the tyrosine kinase inhibitor sunitinib. Front Pharmacol 10:914
Sun L, Trajkovski M (2014) MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism 63(2):272–282
Zou B et al (2015) Persimmon tannin represses 3T3-L1 preadipocyte differentiation via up-regulating expression of miR-27 and down-regulating expression of peroxisome proliferator-activated receptor-γ in the early phase of adipogenesis. Eur J Nutr 54(8):1333–1343
Zhang L-Y et al (2019) MiR-27a promotes EMT in ovarian cancer through active Wnt/?-catenin signalling by targeting FOXO1. Cancer Biomark 24(1):31–42
Min S et al (2012) TGF-β-associated miR-27a inhibits dendritic cell-mediated differentiation of Th1 and Th17 cells by TAB3, p38 MAPK, MAP2K4 and MAP2K7. Genes Immun 13(8):621–631
Lu M et al (2018) miR-149 promotes the myocardial differentiation of mouse bone marrow stem cells by targeting Dab2. Mol Med Rep 17(6):8502–8509
Fomison-Nurse I et al (2018) Diabetes induces the activation of pro-ageing miR-34a in the heart, but has differential effects on cardiomyocytes and cardiac progenitor cells. Cell Death Differ 25(7):1336–1349
Taheri F et al (2020) Regulatory and immunomodulatory role of miR-34a in T cell immunity. Life Sci 262:118209
Shirasaki T et al (2013) MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol 87(9):5270–5286
Bao Y et al (2021) miRNA-27a transcription activated by c-Fos regulates myocardial ischemia-reperfusion injury by targeting ATAD3a. Oxidative Med Cell Longev. https://doi.org/10.1155/2021/2514947
Zhang XL, An BF, Zhang GC (2019) MiR-27 alleviates myocardial cell damage induced by hypoxia/reoxygenation via targeting TGFBR1 and inhibiting NF-kappaB pathway. Kaohsiung J Med Sci 35(10):607–614
Chinchilla A et al (2011) MicroRNA profiling during mouse ventricular maturation: a role for miR-27 modulating Mef2c expression. Cardiovasc Res 89(1):98–108
Liu J-Y et al (2019) Protective effect of down-regulated microRNA-27a mediating high thoracic epidural block on myocardial ischemia-reperfusion injury in mice through regulating ABCA1 and NF-κB signaling pathway. Biomed Pharmacother 112:108606
Cao X et al (2021) MiR-27a-3p/Hoxa10 axis regulates angiotensin II-induced cardiomyocyte hypertrophy by targeting Kv4. 3 expression. Front Pharmacol 12:970
Zhang J et al (2019) miR-27a-5p attenuates hypoxia-induced rat cardiomyocyte injury by inhibiting Atg7. Int J Mol Sci 20(10):2418
Roldan V et al (2014) Prognostic role of MIR146A polymorphisms for cardiovascular events in atrial fibrillation. Thromb Haemost 112(4):781–788
Jin ZQ (2021) MicroRNA targets and biomarker validation for diabetes-associated cardiac fibrosis. Pharmacol Res 174:1941
Jeon YJ et al (2013) Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk. Arterioscler Thromb Vasc Biol 33(2):420–430
Lu M et al (2018) miR149 promotes the myocardial differentiation of mouse bone marrow stem cells by targeting Dab2. Mol Med Rep 17(6):8502–8509
Sayed ASM et al (2015) The diagnostic value of circulating microRNAs for middle-aged (40–60-year-old) coronary artery disease patients. Clinics 70:257–263
Nourbakhsh,M.,et al(2016)Association of miR-34a and mir-149 with obesity and insulin resistance in obese children and adolescents. In: 55th annual ESPE. European Society for Paediatric Endocrinology.
Rafiee A et al (2020) Antimicrobial efficacy of a novel antibiotic-eluting injectable platelet-rich fibrin scaffold against a dual-species biofilm in an infected immature root canal model. BioMed Res Int. https://doi.org/10.1155/2020/6623830
Weber M et al (2011) MicroRNA expression profile in CAD patients and the impact of ACEI/ARB. Cardiol Res Pract. https://doi.org/10.4061/2011/532915
Devaux Y et al (2013) A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PloS one 8(8):e70644
Roncarati R et al (2014) Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 63(9):920–927
Wu K et al (2018) Bioinformatic screening for key miRNAs and genes associated with myocardial infarction. FEBS Open Bio 8(6):897–913
Xue S et al (2019) Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol Med 25(1):1–12
Alisi A et al (2011) Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest 91(2):283–293
Lin Q et al (2009) A role of miR-27 in the regulation of adipogenesis. FEBS J 276(8):2348–2358
Sung JH et al (2016) miRNA polymorphisms (miR146a, miR149, miR196a2 and miR499) are associated with the risk of coronary artery disease. Mol Med Rep 14(3):2328–2342
Wang X et al (2013) Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc Res 97(3):432–442
Feng B et al (2017) miR-146a mediates inflammatory changes and fibrosis in the heart in diabetes. J Mol Cell Cardiol 105:70–76
Ali Sheikh MS et al (2015) Circulating miR-765 and miR-149: potential noninvasive diagnostic biomarkers for geriatric coronary artery disease patients. BioMed Res Int. https://doi.org/10.1155/2015/740301
Van Rooij E et al (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci 105(35):13027–13032
Wu C et al (2013) The human MTHFR rs4846049 polymorphism increases coronary heart disease risk through modifying miRNA binding. Nutr Metab Cardiovasc Dis 23(7):693–698
Chang X et al (2014) Ethnic differences in microRNA-375 expression level and DNA methylation status in type 2 diabetes of Han and Kazak populations. J Diabetes Res. https://doi.org/10.1155/2014/761938
Huang RS et al (2011) Population differences in microRNA expression and biological implications. RNA Biol 8(4):692–701
Han H et al (2015) MiR-34a, miR-21 and miR-23a as potential biomarkers for coronary artery disease: a pilot microarray study and confirmation in a 32 patient cohort. Exp Mol Med 47(2):e138–e138
Gatsiou A et al (2021) Additive contribution of microRNA-34a/b/c to human arterial ageing and atherosclerosis. Atherosclerosis 327:49–58
Ghasempour G et al (2021) Correlations between vitronectin, miR-520, and miR-34 in patients with stenosis of coronary arteries. Mol Biol Rep 48(12):7913–7920
Lee J et al (2010) A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem 285(17):12604–12611
Sabahi Z et al (2020) Syringic acid improves oxidative stress and mitochondrial biogenesis in the liver of streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 10(3):111
Purushotham A et al (2009) Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9(4):327–338
Acknowledgements
The authors’ appreciate Yasuj the University of Medical Sciences for its financial support of this study.
Author information
Authors and Affiliations
Contributions
Dr. AM conceived and designed the evaluation and drafted the manuscript. Dr. EH participated in designing the evaluation, performed parts of the statistical analysis, and helped to draft the manuscript. SH re-evaluated the data, revised the manuscript, and collected the clinical data and Study supervision FS. Interpreted them and revised the manuscript BK. Re-analyzed the clinical, material support, and statistical data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Ethics approval this study was approved by Iran National Committee for Ethics in Biomedical Research (IR.YUMS.REC.1396.121).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosseinpor, S., Khalvati, B., Safari, F. et al. The association of plasma levels of miR-146a, miR-27a, miR-34a, and miR-149 with coronary artery disease. Mol Biol Rep 49, 3559–3567 (2022). https://doi.org/10.1007/s11033-022-07196-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07196-5