Abstract
Background
The enhancement of fish immune system and growth performance throughout the administration of bio-friendly agents such as diet supplements (taurine) is considered a promising alternative in farmed aquatic species.
Materials and methods
The present study was aimed to examine the effect of supplementation of dietary taurine (0, 5 g-TAU5 and 10 g-TAU10) in crystalline form (99% purity) in L. calcarifer juveniles, taking into account growth performance, general health indices and immune related-genes mRNA transcript abundance.
Results
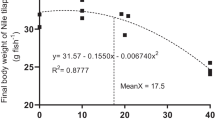
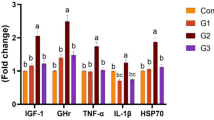
The results confirmed that the supplementation of taurine enhances significantly all the growth parameters and a better flesh quality. While the blood biochemical and immunological factors didn’t present any significant differences, the expression of growth-related genes showed that IGF-1 was almost 3 times higher in fishes fed diet Tau 5 and Tau 10 compared to the control group.
Conclusions
Finally, it can be concluded that at the maximum dose tested (10 g) the treatment was effective for Asian seabass. In addition, Tau inclusion in an FM-based diet can increase the productivity parameters along with raising the antioxidant status.


Similar content being viewed by others
References
Ahmadifar E, Pourmohammadi Fallah H, Yousefi M, Dawood MA, Hoseinifar SH, Adineh H, Yilmaz S, Paolucci M, Doan HV (2021) The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals 11(8):21–67
FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action. FAO, Rome. https://doi.org/10.4060/ca9229en
El-Sayed A-FM (2014) Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev Aquacult 6:241–255
Kim SK, Takeuchi T, Akimoto A, Furuita H, Yamamoto T, Yokoyama M, Murata Y (2005) Effect of taurine supplemented practical diet on growth performance and taurine contents in whole body and tissues of juvenile Japanese flounder Paralichthys olivaceus. Fish Sci 71:627–632
Kim SK, Matsunari H, Takeuchi T, Yokoyama M, Furuita H, Murata Y, Goto T (2008) Comparison of taurine biosynthesis ability between juveniles of Japanese flounder and common carp. Amino Acids 35:161–168
Hardy RW (1995) Nutrition and utilization technology in aquaculture. AOCS Press, Champaign, IL
Salze G, Davis DA (2015) Taurine: a critical nutrient for future fish feeds. Aquaculture 437:215–229. https://doi.org/10.1016/j.aquaculture.2014.12.006
Johnson RB, Kim S-K, Watson AM, Barrows FT, Kroeger EL, Nicklason PM, Goetz GW, Place AR (2015) Effects of dietary taurine supplementation on growth, feed efficiency, and nutrient composition of juvenile sablefish (Anoplopoma fimbria) fed plant based feeds. Aquaculture 445:79–85
Liu Y, Yang P, Hu H, Li Y, Dai J, Zhang Y (2018) The tolerance and safety assessment of taurine as additive in a marine carnivorous fish, Scophthalmus maximus L. Aquacult Nutr 24(1):461–471
Koven W, Peduel A, Gada M, Nixon O, Ucko M (2016) Taurine improves the performance of white grouper juveniles (Epinephelus Aeneus) fed a reduced fish meal diet. Aquaculture 460:8–14
Martins N, Estevão-Rodrigues T, Diógenes AF, Diaz-Rosales P, Oliva-Teles A, Peres H (2018) Taurine requirement for growth and nitrogen accretion of European seabass (Dicentrarchus labrax, L.) juveniles. Aquaculture 494:19–25. https://doi.org/10.1016/j.aquaculture.2018.05.007
De Moura LB, Diógenes AF, Campelo DAV, De Almeidak FLA, Pousão-Ferreira PM, Furuya WM, Oliva-Teles A, Peres H (2018) Taurine and methionine supplementation as a nutritional strategy for growth promotion of meagre (Argyrosomus regius) fed high plant protein diets. Aquaculture 497:389–395. https://doi.org/10.1016/j.aquaculture.2018.07.038
Cheng CH, Guo ZX, Wang AL (2018) The protective effects of taurine on oxidative stress, cytoplasmic free-Ca2+ and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol 77:457–464. https://doi.org/10.1016/j.fsi.2018.04.022
Sampath H, Group GBW, Rathnayake RMDS, Yangm M, Zhang W (2020) Roles of dietary taurine in fish nutrition. Mar Life Sci Technol 2:360–375. https://doi.org/10.1007/s42995-020-00051-1
Ashouri G, Soofiani NM, Hoseinifar SH, Jalali SAH, Morshedi V, Van Doan H, Mozanzadeh MT (2018) Combined effects of dietary low molecular weight sodium alginate and Pediococcus acidilactici MA18/5 M on growth performance, haematological and innate immune responses of Asian seabass (Lates calcalifer) juveniles. Fish Shellfish Immunol 79:34–41
AOAC (2005) Official method of analysis, 18th edn. Association of Officiating Analytical Chemists, Washington, DC
Regoli F, Bocchetti R, Filho DW (2012) Spectrophotometric assays of antioxidants. In: Abele D, Vázquez-Medina JP, Zenteno-Savín T (eds) Oxidative stress in aquatic ecosystems. Wiley, Chichester, pp 367–380
Cohen MB, Duvel DL (1988) Characterization of the inhibition of glutathione reductase and the recovery of enzyme activity in exponentially growing murine leukemia (11210) cells treated with 1, 3-bis (2-chloroethyl)-1-nitrosourea. Biochem Pharmacol 37:3317–3320
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Hoseinifar SH, Sohrabi A, Paknejad H, Jafari V, Paolucci M, Van Doan H (2019) Enrichment of common carp (Cyprinus carpio) fingerlings diet with Psidium guajava: the effects on cutaneous mucosal and serum immune parameters and immune related genes expression. Fish Shellfish Immunol 86:688–694. https://doi.org/10.1016/j.fsi.2018.12.001
Ellis AE (1990) Lysozyme assays. In: Stolen JS, Fletcher TC, Rowley AF, Zelikoff J, Kaattari S, Smith S (eds) Techniques in fish immunology. SOS Publications, Cambridge, pp 101–103
Siwicki A (1993) Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. In: Olsztyn P (ed) Disease diagnosis and prevention methods. FAO, Rome, pp 105–112
Bagni M, Romano N, Finoia MG, Abelli L, Scapigliati G, Tiscar PG, Sarti M, Marino G (2005) Short-and long-term effects of a dietary yeast β-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol 18(4):311–325
Zeynali M, Nafisi Bahabadi M, Morshedi V, Ghasemi A, Torfi Mozanzadeh M (2020) Replacement of dietary fishmeal with Sargassum ilicifolium meal on growth, innate immunity and immune gene mRNA transcript abundance in Lates calcarifer juveniles. Aquacult Nutr 26(5):1657–1668
Kato K, Yamamoto M, Peerapon K, Fukada H, Biswas A, Yamamoto S, Takii K, Miyashita S (2014) Effects of dietary taurine levels on epidermal thickness and scale loss in red sea bream, Pagrus major. Aquacullt Res 45(11):1818–1824
Saleh NE, Wassef EA, Ashry AM (2020) Is a taurine supplement necessary in fishmeal-based feeds for juvenile European sea bass (Dicentrarchus labrax)? Aquacult Int 28:321–333. https://doi.org/10.1007/s10499-019-00464-5
Martinez JB, Chatzifotis S, Divanach P, Takeuchi T (2004) Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus labrax fry fed with demand-feeders. Fish Sci 70:74–79
Matsunari H, Yamamoto T, Kim SK, Goto T, Takeuchi T (2008) Optimum dietary taurine level in casein-based diet for juvenile red sea bream Pagrus major. Fish Sci 74:347–353
Espe M, Ruohonen K, El-Mowafi A (2012) Effect of taurine supplementation on the metabolism and body lipid-to-protein ratio in juvenile Atlantic salmon (Salmo salar). Aquacult Res 43:349–360
Li M, Lai H, Li Q, Gong S, Wang R (2016) Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus fulvidraco fed all-plant protein diets. Aquaculture 450:349–355
Yun B, Ai Q, Mai K, Xu W, Qi G, Luo Y (2012) Synergistic effects of dietary cholesterol and taurine on growth performance and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed high plant protein diets. Aquaculture 324:85–91
Qi G, Ai Q, Mai K, Xu W, Liufu Z, Yun B, Zhou H (2012) Effects of dietary taurine supplementation to a casein-based diet on growth performance and taurine distribution in two sizes of juvenile turbot (Scophthalmus maximus L.). Aquaculture 358:122–128
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Han YZ, Koshio S, Jiang ZQ, Ren TJ, Ishikawa M, Yokoyama S, Gao J (2014) Interactive effects of dietary taurine and glutamine on growth performance, blood parameters and oxidative status of Japanese flounder Paralichthys olivaceus. Aquaculture 434:348–354
Kim J-M, Malintha GHT, Gunathilaka GLBE, Lee C, Kim M-G, Lee B-J, Kim J-D, Lee K-J (2017) Taurine supplementation in diet for olive flounder at low water temperature. Fish Aquat Sci 20(1):1–8
Dehghani R, Oujifard A, Mozanzadeh MT, Morshedi V, Bagheri D (2020) Effects of dietary taurine on growth performance, antioxidant status, digestive enzymes activities and skin mucosal immune responses in yellowfin seabream, Acanthopagrus latus. Aquaculture 517:734–795
Gunathilaka GLBE, Kim M-G, Lee C, Shin J, Lee BJ, Lee K-J (2019) Effects of taurine supplementation in low fish meal diets for red seabream (Pagrus major) in low water temperature season. Fish Aquat Sci 22:1–10
Duan C (1998) Nutritional and developmental regulation of insulin-like growth factors in fish. J Nutr 128:3056–3145
Vanitha MK, Priya KD, Baskaran K, Periyasamy K, Saravanan D, Venkateswari R, Mani BR, Ilakkia A, Selvaraj S, Menaka R, Geetha M, Rashanthy N, Anandakumar P, Sakthisekaran D (2015) Taurine regulates mitochondrial function during 7,12-dimethyl benz[a]anthracene induced experimental mammary carcinogenesis. J Pharmacopuncture 18(3):68–74. https://doi.org/10.3831/KPI.2015.18.027
Fang YZ, Sheng Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18(10):872–879. https://doi.org/10.1016/s0899-9007(02)00916-4
Biller JD, Takahashi LS (2018) Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity. An Acad Bras Ciênc 90(4):3403–3414. https://doi.org/10.1590/0001-3765201820170730
Acknowledgements
We are grateful to the director and staff of Hounam Abziparvaran Naji Company, Shiraz, Iran for providing the necessary facilities for the experiment.
Author information
Authors and Affiliations
Contributions
All persons listed as authors have read, contributed to preparing the manuscript as given below: SH and DB: fish maintenance, sample collection and some analyses; VM: experimental design, wrote the manuscript and statistical analyses; AG: gene expression analyses; SR: wrote the manuscript; RG: data interpretation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morshedi, V., Rainis, S., Hamedi, S. et al. Effects of dietary taurine amino acid on growth performance, mucosal and immune response, gene expression and antioxidant defence of asian seabass (Lates calcarifer). Mol Biol Rep 49, 3503–3510 (2022). https://doi.org/10.1007/s11033-022-07187-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07187-6