Abstract
Background
The increasing need for therapeutic monoclonal antibodies (mAbs) entails the development of innovative and improved expression strategies. Chromatin insulators have been utilized for the enhancement of the heterologous proteins in mammalian cells.
Methods and results
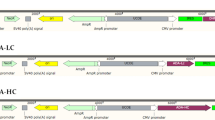
In the current study the Ccnb1ip1 gene insulator element was utilized to construct a novel vector system for the expression of an anti-CD52 mAb in Chinese hamster ovary (CHO) cells. The insulator containing (pIns-mAb) and control (pmAb) vectors were generated and stable cell pools were established using these constructs. The expression level in the cells created with pIns-mAb vector was calculated to be 233 ng/mL, and the expression rate in the control vector was 210 ng/mL, which indicated a 10.9% increase in mAb expression in pIns-mAb pool. In addition, analysis of mAb expression in clonal cells established from each pool showed a 10% increase in antibody productivity in the highest mAb producing clone derived from the pIns-mAb pool compared to the clone isolated from pmAb pool.
Conclusions
More studies are needed to fully elucidate the effects of Ccnb1ip1 gene insulator on recombinant therapeutic protein expression in mammalian cells. The combination of this element with other chromatin-modifying elements might improve its augmentation effect which could pave the way for efficient and cost-effective production of therapeutic drugs.





Similar content being viewed by others
References
Ecker DM, Jones SD, Levine HL (2015) The therapeutic monoclonal antibody market. MAbs 7:9–14
Walsh G (2018) Biopharmaceutical benchmarks 2018. Nat Biotechnol 36:1136–1145
Kim JY, Kim Y-G, Lee GM (2012) CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol 93:917–930
Kunert R, Reinhart D (2016) Advances in recombinant antibody manufacturing. Appl Microbiol Biotechnol 100:3451–3461
Lai T, Yang Y, Ng SK (2013) Advances in mammalian cell line development technologies for recombinant protein production. Pharmaceuticals 6:579–603
Ali T, Renkawitz R, Bartkuhn M (2016) Insulators and domains of gene expression. Curr Opin Genet Dev 37:17–26
Yoshida W, Tomikawa J, Inaki M, Kimura H, Onodera M, Hata K, Nakabayashi K (2015) An insulator element located at the cyclin B1 interacting protein 1 gene locus is highly conserved among mammalian species. PLoS ONE 10:e0131204
Lill CM (2014) Recent advances and future challenges in the genetics of multiple sclerosis. Front Neurol 5:130
Deiß A, Brecht I, Haarmann A, Buttmann M (2013) Treating multiple sclerosis with monoclonal antibodies: a 2013 update. Expert Rev Neurother 13:313–335
Pairawan MS, Bolhassani A, Rahimpour A (2019) Enhanced transient expression of an anti-CD52 monoclonal antibody in CHO cells through utilization of miRNA sponge technology. Res Pharm Sci 14:335–342
Mohammadian O, Rajabibazl M, Pourmaleki E, Bayat H, Ahani R, Rahimpour A (2019) Development of an improved lentiviral based vector system for the stable expression of monoclonal antibody in CHO cells. Prep Biochem Biotechnol 49:822–829
Adams GP, Weiner LM (2005) Monoclonal antibody therapy of cancer. Nat Biotechnol 23:1147–1157
Rajabibazl M, Rasaee MJ, Forouzandeh M, Rahimpour A (2013) Retroviral transduction of fluonanobody and the variable domain of camelid heavy-chain antibodies to chicken embryonic cells. Iran J Immunol 10:247–258
Minagar A (2013) Current and future therapies for multiple sclerosis. Scientifica. https://doi.org/10.1155/2013/249101
Ravandi F, O’Brien S (2005) Alemtuzumab. Expert Rev Anticancer Ther 5:39–51
Havrdova E, Horakova D, Kovarova I (2015) Alemtuzumab in the treatment of multiple sclerosis: key clinical trial results and considerations for use. Ther Adv Neurol Disord 8:31–45
Naderi F, Hashemi M, Bayat H, Mohammadian O, Pourmaleki Eh, Etemadzadeh MH, Rahimpour A (2018) The augmenting effects of the tDNA insulator on stable expression of monoclonal antibody in Chinese hamster ovary cells. Monoclon Antib Immunodiagn Immunother 37:200–206
Kim J-M, Kim J-S, Park D-H, Kang HS, Yoon J, Baek K, Yoon Y (2004) Improved recombinant gene expression in CHO cells using matrix attachment regions. J Biotechnol 107:95–105
Wang F, Wang T-Y, Tang Y-Y, Zhang J-H, Yang X-J (2012) Different matrix attachment regions flanking a transgene effectively enhance gene expression in stably transfected Chinese hamster ovary cells. Gene 500:59–62
Toby GG, Gherraby W, Coleman TR, Golemis EA (2003) A novel RING finger protein, human enhancer of invasion 10, alters mitotic progression through regulation of cyclin B levels. Mol Cell Biol 23:2109–2122
Ward JO, Reinholdt LG, Motley WW, Niswander LM, Deacon DC, Griffin LB, Langlais KK, Backus VL, Schimenti KJ, O’Brien MJ (2007) Mutation in mouse hei10, an e3 ubiquitin ligase, disrupts meiotic crossing over. PLoS Genet 3:e139
Grönholm M, Muranen T, Toby G, Utermark T, Hanemann C, Golemis E, Carpen O (2006) A functional association between merlin and HEI10, a cell cycle regulator. Oncogene 25:4389–4398
Mine N, Kurose K, Konishi H, Araki T, Nagai H, Emi M (2001) Fusion of a Sequence from HEI10 (14q11) to the HMGIC gene at 12ql5 in a uterine leiomyoma. Jpn J Cancer Res 92:135–139
Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22:1393–1398
Jayapal KP, Wlaschin KF, Hu W, Yap MG (2007) Recombinant protein therapeutics from CHO cells-20 years and counting. Chem Eng Prog 103:40
Payandeh Z, Rajabibazl M, Mortazavi Y, Rahimpour A, Taromchi AH, Dastmalchi S (2019) Affinity maturation and characterization of the ofatumumab monoclonal antibody. J Cell Biochem 120:940–950
Thomas CE, Ehrhardt A, Kay MA (2003) Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4:346–358
Ramamoorth M, Narvekar A (2015) Non viral vectors in gene therapy-an overview. J Clin Diagn Res. https://doi.org/10.7860/JCDR/2015/10443.5394
Mahboudi S, Moosavi-Nasab M, Kazemi B, Rahimpour A, Eskandari MH, Mohammadian O, Shams F (2021) Utilization of the human gamma-satellite insulator for the enhancement of anti-PCSK9 monoclonal antibody expression in Chinese hamster ovary cells. Mol Biol Rep 48:4405–4412
Acknowledgements
The authors wish to thank Medical Nano-Technology & Tissue Engineering Research Center and School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Funding
This study was funded by the “the Arak University of Medical Sciences” (Grant Number: 3150).
Author information
Authors and Affiliations
Contributions
Authors participated in conception and design (AR, MG, EP and MD), data collection and statistical analysis (AR, MG, FS, ZP and NP), performing experiments (AR, EP, FS, ZP, NP, MD and MG), and drafting the article or revising critically for important intellectual content (AR, EP, FS, ZP, NP, MD and MG).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee at Arak University of Medical Sciences (IR.ARAKMU.REC.1397.128).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahimpour, A., Pourmaleki, E., Shams, F. et al. The effect of Ccnb1ip1 insulator on monoclonal antibody expression in Chinese hamster ovary cells. Mol Biol Rep 49, 3461–3468 (2022). https://doi.org/10.1007/s11033-022-07182-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07182-x