Abstract
Background
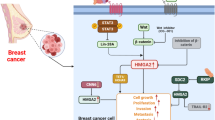
Breast cancer (BC) is the most common malignancy in females and is the second leading cause of cancer-related death among women worldwide. Midkine (MDK) is a heparin-binding growth factor that is abnormally expressed at high levels in various human malignancies. We aimed to uncover the biological function and molecular mechanism of MDK in BC cells.
Methods and results
MDA-MB-231-shMDK and T47D-shMDK BC cells were established. The in vitro biological functions of MDK were demonstrated by CCK-8 assays, Transwell assays and Western blotting, whereas qPCR pathway arrays were implemented to explore the mechanism of MDK in BC cells. Functionally, we verified that silencing MDK significantly suppressed BC cell proliferation and migration by inhibiting the activation of the nuclear factor kappa B (NF-κB) pathway and the nuclear distribution of NF-κB. Meanwhile, Ingenuity Pathway Analysis (IPA) and a qPCR pathway array revealed that silencing MDK decreased the expression of NR3C1, a potential downstream target of the NF-κB pathway. We also confirmed that treatment with an NF-κB inhibitor suppressed NR3C1 expression in BC cells. Finally, we demonstrated that silencing NR3C1 repressed BC cell proliferation and migration.
Conclusions
Our findings highlight a novel mechanism by which MDK influences BC progression via regulation of the NF-κB-NR3C1 pathway.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30. https://doi.org/10.3322/caac.21590
Fahad UM (2019) Breast cancer: current perspectives on the disease status. Adv Exp Med Biol 1152:51–64. https://doi.org/10.1007/978-3-030-20301-6_4
Tyanova S, Albrechtsen R, Kronqvist P, Cox J, Mann M, Geiger T (2016) Proteomic maps of breast cancer subtypes. Nat Commun 7:10259. https://doi.org/10.1038/ncomms10259
Harbeck N, Gnant M (2017) Breast cancer. Lancet 389:1134–1150. https://doi.org/10.1016/s0140-6736(16)31891-8
Kadomatsu K, Muramatsu T (2004) Midkine and pleiotrophin in neural development and cancer. Cancer Lett 204:127–143. https://doi.org/10.1016/s0304-3835(03)00450-6
Filippou PS, Karagiannis GS, Constantinidou A (2020) Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene 39:2040–2054. https://doi.org/10.1038/s41388-019-1124-8
Shin DH, Jo JY, Kim SH, Choi M, Han C, Choi BK, Kim SS (2020) Midkine is a potential therapeutic target of tumorigenesis, angiogenesis, and metastasis in non-small cell lung cancer. Cancers (Basel) 12:2402. https://doi.org/10.3390/cancers12092402
Jones DR (2014) Measuring midkine: the utility of midkine as a biomarker in cancer and other diseases. Br J Pharmacol 171:2925–2939. https://doi.org/10.1111/bph.12601
Kemper M, Hentschel W, Graß JK, Stüben BO, Konczalla L, Rawnaq T, Ghadban T, Izbicki JR, Reeh M (2020) Serum midkine is a clinical significant biomarker for colorectal cancer and associated with poor survival. Cancer Med 9:2010–2018. https://doi.org/10.1002/cam4.2884
López-Valero I, Dávila D, González-Martínez J et al (2020) Midkine signaling maintains the self-renewal and tumorigenic capacity of glioma initiating cells. Theranostics 10:5120–5136. https://doi.org/10.7150/thno.41450
Ibusuki M, Fujimori H, Yamamoto Y, Ota K, Ueda M, Shinriki S, Taketomi M, Sakuma S, Shinohara M, Iwase H, Ando Y (2009) Midkine in plasma as a novel breast cancer marker. Cancer Sci 100:1735–1739. https://doi.org/10.1111/j.1349-7006.2009.01233.x
Li F, Tian P, Zhang J, Kou C (2015) The clinical and prognostic significance of midkine in breast cancer patients. Tumour Biol 36:9789–9794. https://doi.org/10.1007/s13277-015-3710-x
Gharesouran J, Taheri M, Sayad A, Ghafouri-Fard S, Mazdeh M, Omrani MD (2018) The growth arrest-specific transcript 5 (GAS5) and nuclear receptor subfamily 3 group c member 1 (NR3C1): Novel markers involved in multiple sclerosis. Int J Mol Cell Med 7:102–110. https://doi.org/10.22088/IJMCM.BUMS.7.2.102
Han Z, Zhang C, Wang Q, Li L, Wang M, Li X, Yang C (2021) MicroRNA-19b downregulates NR3C1 and enhances oxaliplatin chemoresistance in colon cancer via the PI3K/AKT/mTOR pathway. Clin Med Insights Oncol 15:11795549211012666. https://doi.org/10.1177/11795549211012666
Chen X, Chen F, Ren Y, Weng G, Keng PC, Chen Y, Lee SO (2019) Glucocorticoid receptor upregulation increases radioresistance and triggers androgen independence of prostate cancer. Prostate 79:1386–1398. https://doi.org/10.1002/pros.23861
Lovšin N, Marc J (2021) Glucocorticoid receptor regulates TNFSF11 transcription by binding to glucocorticoid responsive element in TNFSF11 proximal promoter region. Int J Mol Sci 22:1054. https://doi.org/10.3390/ijms22031054
Chen Z, Lan X, Wu D, Sunkel B, Ye Z, Huang J, Liu Z, Clinton SK, Jin VX, Wang Q (2015) Ligand-dependent genomic function of glucocorticoid receptor in triple-negative breast cancer. Nat Commun 6:8323. https://doi.org/10.1038/ncomms9323
Pan D, Kocherginsky M, Conzen SD (2011) Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res 71:6360–6370. https://doi.org/10.1158/0008-5472.can-11-0362
He L, Yuan L, Sun Y et al (2019) Glucocorticoid receptor signaling activates TEAD4 to promote breast cancer progression. Cancer Res 79:4399–4411. https://doi.org/10.1158/0008-5472.can-19-0012
Weckbach LT, Grabmaier U, Uhl A et al (2019) Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J Exp Med 216:350–368. https://doi.org/10.1084/jem.20181102
Sakamoto K, Kadomatsu K (2012) Midkine in the pathology of cancer, neural disease, and inflammation. Pathol Int 62:445–455. https://doi.org/10.1111/j.1440-1827.2012.02815.x
Yuan K, Chen Z, Li W, Gao CE, Li G, Guo G, Yang Y, Ai Y, Wu L, Zhang M (2015) MDK protein overexpression correlates with the malignant status and prognosis of non-small cell lung cancer. Arch Med Res 46:635–641. https://doi.org/10.1016/j.arcmed.2015.11.006
Tian W, Shen J, Chen W (2017) Suppression of midkine gene promotes the antitumoral effect of cisplatin on human gastric cancer cell line AGS in vitro and in vivo via the modulation of notch signaling pathway. Oncol Rep 38:745–754. https://doi.org/10.3892/or.2017.5743
Rawnaq T, Dietrich L, Wolters-Eisfeld G, Uzunoglu FG, Vashist YK, Bachmann K, Simon R, Izbicki JR, Bockhorn M, Güngör C (2014) The multifunctional growth factor midkine promotes proliferation and migration in pancreatic cancer. Mol Cancer Res 12:670–680. https://doi.org/10.1158/1541-7786.mcr-13-0467
Erdogan S, Turkekul K, Dibirdik I, Doganlar O, Doganlar ZB, Bilir A, Oktem G (2018) Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed Pharmacother 107:793–805. https://doi.org/10.1016/j.biopha.2018.08.061
Erdogan S, Turkekul K, Dibirdik I, Doganlar ZB, Doganlar O, Bilir A (2020) Midkine silencing enhances the anti-prostate cancer stem cell activity of the flavone apigenin: cooperation on signaling pathways regulated by ERK, p38, PTEN, PARP, and NF-κB. Invest New Drugs 38:246–263. https://doi.org/10.1007/s10637-019-00774-8
Zhang Y, Meng Z, Zhang M et al (2014) Immunohistochemical evaluation of midkine and nuclear factor-kappa B as diagnostic biomarkers for papillary thyroid cancer and synchronous metastasis. Life Sci 118:39–45. https://doi.org/10.1016/j.lfs.2014.09.025
Kuo AH, Stoica GE, Riegel AT, Wellstein A (2007) Recruitment of insulin receptor substrate-1 and activation of NF-kappaB essential for midkine growth signaling through anaplastic lymphoma kinase. Oncogene 26:859–869. https://doi.org/10.1038/sj.onc.1209840
Veneris JT, Huang L, Churpek JE, Conzen SD, Fleming GF (2019) Glucocorticoid receptor expression is associated with inferior overall survival independent of BRCA mutation status in ovarian cancer. Int J Gynecol Cancer 29:357–364. https://doi.org/10.1136/ijgc-2018-000101
Veneris JT, Darcy KM, Mhawech-Fauceglia P, Tian C, Lengyel E, Lastra RR, Pejovic T, Conzen SD, Fleming GF (2017) High glucocorticoid receptor expression predicts short progression-free survival in ovarian cancer. Gynecol Oncol 146:153–160. https://doi.org/10.1016/j.ygyno.2017.04.012
Tangen IL, Veneris JT, Halle MK, Werner HM, Trovik J, Akslen LA, Salvesen HB, Conzen SD, Fleming GF, Krakstad C (2017) Expression of glucocorticoid receptor is associated with aggressive primary endometrial cancer and increases from primary to metastatic lesions. Gynecol Oncol 147:672–677. https://doi.org/10.1016/j.ygyno.2017.09.013
Lamb CA, Vanzulli SI, Lanari C (2019) Hormone receptors in breast cancer: more than estrogen receptors. Medicina (B Aires) 79:540–545
Shi W, Wang D, Yuan X, Liu Y, Guo X, Li J, Song J (2019) Glucocorticoid receptor-IRS-1 axis controls EMT and the metastasis of breast cancers. J Mol Cell Biol 11:1042–1055. https://doi.org/10.1093/jmcb/mjz001
Conway ME, McDaniel JM, Graham JM, Guillen KP, Oliver PG, Parker SL, Yue P, Turkson J, Buchsbaum DJ, Welm BE, Myers RM, Varley KE (2020) STAT3 and GR cooperate to drive gene expression and growth of basal-like triple-negative breast cancer. Cancer Res 80:4355–4370. https://doi.org/10.1158/0008-5472.can-20-1379
Sorrentino G, Ruggeri N, Zannini A et al (2017) Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun 8:14073. https://doi.org/10.1038/ncomms14073
Funding
This work was supported by the National Natural Science Foundation of China (No. 82002919), Jiangsu Province Key Laboratory of Immunity and Metabolism Open Project Fund (JSKIM201803), Xuzhou Science and Technology Project (KC20101, KC20070) and Jiangsu Province Key Laboratory of Anesthesiology Open Project Fund (XZSYSKF2019023).
Author information
Authors and Affiliations
Contributions
LZ and QW proposed the hypotheses and designed the research; LZ, YX performed the experiments; YX and PZ participated in the analysis of the results; LS and MZ edited the pictures; LS revised the manuscript; QW supervised research and Finalize the final draft.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Song, L., Xu, Y. et al. Midkine promotes breast cancer cell proliferation and migration by upregulating NR3C1 expression and activating the NF-κB pathway. Mol Biol Rep 49, 2953–2961 (2022). https://doi.org/10.1007/s11033-022-07116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07116-7