Abstract
Background
The aim of this study was to study the relationship between the methylation level of the promoter region of follicle-stimulating hormone receptor (FSHR) gene and the mRNA expression of Duolang sheep fed different energy diets.
Methods
In this study, polyembryo estrus Duolang sheep under different energy levels were selected as the experimental subjects. Dietary nutrient level reference (NY/T 816-2004), medium energy level was 10.88 MJ/day, high and low energy groups were increased and decreased by 15% on the basis of medium energy level, respectively 12.51 MJ/day, 9.25 MJ/day. Through RNA and DNA extraction, qPCR, bisulfitegenomicse-quencing PCR (BSP), sequence matching and other analysis of ovarian tissue of Duolang sheep. The difference of DNA methylation level and mRNA expression of FSHR gene during estrus in Duolang sheep fed with different energy diets was detected.
Results
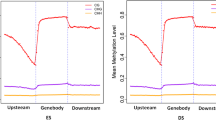
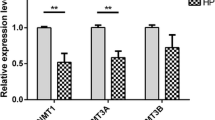
The results showed the expression level of FSHR in high energy group was significantly higher than that in low energy group (P < 0.01), the expression level of FSHR in high energy group was significantly higher than that in medium energy group (P < 0.05), and the expression level of FSHR in medium energy group was significantly higher than that in low energy group (P < 0.05). In the target fragment of the promoter region of the FSHR gene, the methylation rate was 25% in the high energy group, 50% in the normal group, and 75% in the low energy group.
Conclusions
This study revealed that different dietary energy levels had certain effects on the FSHR gene DNA methylation level and mRNA expression, and the expression level was negatively correlated with methylation level.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
Le LSNZ, Hong-cai CJS (2021) Effects of different FSH treatments on synchronous estrus of Duolang sheep. Grass-Feeding Livest (02):20–24. https://doi.org/10.16863/j.cnki.1003-6377.2021.02.004
Yunxia G (2018) Effect of short-term nutritional supplementation on folliculogenesis and its mechanism of regulation in sheep during the luteal phase. Hebei Agricultural University.05–28
Suleman A (2012) Polymorphism of several candidate genes related to reproductive traits of Duolang sheep. Xinjiang Agricultural University. 05–01
Epperson LE, Martin SL (20020). Quantitative assessment of ground squirrel mRNA levels in multiple stages of hibernation. Physiol Genomics 10(2):93–102. https://doi.org/10.1152/physiolgenomics.00004.2002
Law P, Holland ML (2019) DNA methylation at the crossroads of gene and environment interactions. Essays Biochem 63(6):717–726. https://doi.org/10.1042/EBC20190031
Yang C, Ye J, Li X, Gao X, Zhang K, Luo L, Ding J, Zhang Y, Li Y, Cao H, Ling Y, Zhang X, Liu Y, Fang F (2016) DNA methylation patterns in the hypothalamus of female pubertal goats. PLoS ONE 11(10):e165327. https://doi.org/10.1371/journal.pone.0165327
Lomniczi A, Wright H, Ojeda SR (2015) Epigenetic regulation of female puberty. Front Neuroendocrin 36:90–107. https://doi.org/10.1016/j.yfrne.2014.08.003
Alvarado S, Fernald RD, Storey KB, Szyf M (2014) The dynamic nature of DNA methylation: a role in response to social and seasonal variation. Integr Comp Biol 54(1):68–76. https://doi.org/10.1093/icb/icu034
L Zaiping (2002) Molecular cloning experimental guidelines, 3rd edn. Scientific Bulletin (24):1–1888
LNWZ, Jia C (2005) Expression of Erα and Pr in estrus of small tail han sheep with different BMPrib genotypes. Chin J Anim Sci (11):30–33
Kiyma Z, Alexander BM, Van Kirk EA, Murdoch WJ, Hallford DM, Moss GE (2004) Effects of feed restriction on reproductive and metabolic hormones in ewes. J Anim Sci 82(9):2548–2557. https://doi.org/10.2527/2004.8292548x
Ying S, Wang Z, Wang C, Nie H, He D, Jia R, Wu Y, Wan Y, Zhou Z, Yan Y, Zhang Y, Wang F (2011) Effect of different levels of short-term feed intake on folliculogenesis and follicular fluid and plasma concentrations of lactate dehydrogenase, glucose, and hormones in Hu sheep during the luteal phase. Reproduction 142(5):699–710. https://doi.org/10.1530/REP-11-0229
Khlil ZB, Khnissi S, Rekik M, Lassoued N (2017) Feed supplementation improves estrus response and increases fertility of sheep induced to breed out of season. Trop Anim Health PRO 49(3):607–612. https://doi.org/10.1007/s11250-017-1236-5
Kumar D, De K, Shekhawat I, Bahadur S, Balaganur K, Naqvi SMK (2019) Combined effect of heat and nutritional stress on superovulation of Malpura ewes in a semi-arid region. J Therm Biol 80:158–163. https://doi.org/10.1016/j.jtherbio.2019.02.007
Kaminski SL, Redmer DA, Bass CS, Keisler DH, Carlson LS, Vonnahme KA, Dorsam ST, Grazul-Bilska AT (2015) The effects of diet and arginine treatment on serum metabolites and selected hormones during the estrous cycle in sheep. Theriogenology 83(5):808–816. https://doi.org/10.1016/j.theriogenology.2014.11.017
Xinxin JWPX (2005) Effects of dietary energy and protein levels on reproductive performance and blood biochemical indices of Duolang sheep. Chin Herbivores 05:21–25
Oqla HM, Kridli RT, Haddad S (2004) The effect of dietary fat inclusion on nutrient intake and reproductive performance in postpartum Awassi ewes. Asian Aust J Anim Sci 17(10):1395–1399
Keomanivong FE, Grazul-Bilska AT, Redmer DA, Bass CS, Kaminski SL, Borowicz PP, Kirsch JD, Swanson KC (2017) The impact of diet and arginine supplementation on pancreatic mass, digestive enzyme activity and insulin-containing cell cluster morphology during the estrous cycle in sheep. Domest Anim Endocrinol 59:23–29
Webb R, Garnsworthy P, Gong J, Armstrong D (2004) Control of follicular growth: local interactions and nutritional influences. J Anim Sci 82(E-Suppl):E63–E74. https://doi.org/10.2527/2004.8213_supplE63x
Visser JA, Themmen A (2014) Role of anti-Mullerian hormone and bone morphogenetic proteins in the regulation of FSH sensitivity. Mol Cell Endocrinol 382(1):460–465. https://doi.org/10.1016/j.mce.2013.08.012
Yao YC, Song XT, Zhai YF, Liu S, Lu J, Xu X, Qi MY, Zhang JN, Huang H, Liu YF, Liu GS, Yuan H (2021) Transcriptome analysis of sheep follicular development during prerecruitment, dominant, and mature stages after FSH superstimulation. Domest Anim Endocrin 74:106563. https://doi.org/10.1016/j.domaniend.2020.106563
Omari M, Lange A, Plontzke J, Roblitz S (2020) Model-based exploration of the impact of glucose metabolism on the estrous cycle dynamics in dairy cows. Biol Direct 15(1):2. https://doi.org/10.1186/s13062-019-0256-7
Viñoles C, Forsberg M, Martin GB, Cajarville C, Repetto J, Meikle A (2005) Short-term nutritional supplementation of ewes in low body condition affects follicle development due to an increase in glucose and metabolic hormones. Reproduction 129(3):299–309. https://doi.org/10.1530/rep.1.00536
Scaramuzzi RJ, Brown HM, Dupont J (2010) Nutritional and metabolic mechanisms in the ovary and their role in mediating the effects of diet on folliculogenesis: a perspective. Reprod Domest Anim 45(Suppl 3):32–41. https://doi.org/10.1111/j.1439-0531.2010.01662.x
Habibizad J, Riasi A, Kohram H, Rahmani HR (2015) Effect of feeding greater amounts of dietary energy for a short-term with or without eCG injection on reproductive performance, serum metabolites and hormones in ewes. Anim Reprod Sci 160:82–89. https://doi.org/10.1016/j.anireprosci.2015.07.007
YW (2007) Effects of energy source and level on quality and related gene expression of gilt oocytes. Sichuan Agricultural University. 04:56
Zhou X, Yu M, Liu L, Yi K, Li C, Chen L, Sun Y (2009) Effects of dietary energy level on ovarian expression of mRNA s for luteinizing hormone receptor and follicle-stimulatiing hormone receptor in prepubertal gilts. Chin J Vet Sci 1:97–105
Ying SJ, Xiao SH, Wang CL, Zhong BS, Zhang GM, Wang ZY, He DY, Ding XL, Xing HJ, Wang F (2013) Effect of nutrition on plasma lipid profile and mRNA levels of ovarian genes involved in steroid hormone synthesis in Hu sheep during luteal phase1. J Anim Sci 91(11):5229–5239. https://doi.org/10.2527/jas.2013-6450
Choi S, Friso S (2010) Epigenetics: a new bridge between nutrition and health. Adv Nutr 1(1):8–16. https://doi.org/10.3945/an.110.1004
Tanwar VS, Ghosh S, Sati S, Ghose S, Kaur L, Kumar KA, Shamsudheen KV, Patowary A, Singh M, Jyothi V, Kommineni P, Sivasubbu S, Scaria V, Raghunath M, Mishra R, Chandak GR, Sengupta S (2020) Maternal vitamin B12 deficiency in rats alters DNA methylation in metabolically important genes in their offspring. Mol Cell Biochem 468(1–2):83–96. https://doi.org/10.1007/s11010-020-03713-x
Chang G, Zhang K, Xu T, Jin D, Guo J, Zhuang S, Shen X (2015) Epigenetic mechanisms contribute to the expression of immune related genes in the livers of dairy cows fed a high concentrate diet. PLoS ONE 10(4):e123942. https://doi.org/10.1371/journal.pone.0123942
Dong G, Qiu M, Ao C, Zhou J, Khas-Erdene XW, Zhang Z, Yang Y, Andrews Z (2014) Feeding a high-concentrate corn straw diet induced epigenetic alterations in the mammary tissue of dairy cows. PLoS ONE 9(9):e107659. https://doi.org/10.1371/journal.pone.0107659
Acknowledgements
Thanks to the above the author with the help of various aspects. Thanks for the project support of the Efficient Utilization of Tarim Sheep Germplasm Resources (Grant No. 2019CB010).
Author information
Authors and Affiliations
Contributions
BL, conceived, drafted and approved this research article. Conceptualization: QHG; Data curation: BL; formal analysis: BL; funding acquisition: QHG; investigation: BL, FH; methodology: BL, WKT, XR; project administration: QHG, HJL; resources: XR, PN; visualization: BL, XR; roles/writing—original draft: BL; writing—review and editing: JW, PN, FH, DF; all authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests associated with the manuscript.
Ethical approval
All animal experiments were conducted according to the Regulations and Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China, revised in 2004). The present study was approved by the Institutional Animal Care and Use Committee of Tarim University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, B., Li, H., Tao, W. et al. Effects of different energy diets on DNA methylation and mRNA expression in follicle stimulating hormone receptor gene promoter region of Duolang sheep during estrus. Mol Biol Rep 49, 2565–2577 (2022). https://doi.org/10.1007/s11033-021-07058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-07058-6