Abstract
Background
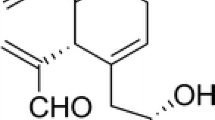
Scutellarein, a widely studied ingredient of scutellaria herbs, has higher bioavailability and solubility than that of scutellarin. Although the scutellarein had been reported to modulate numerous biological functions, its ability in suppressing cardiac hypertrophy remains unclear. Hence, the present study attempted to investigate whether scutellarein played critical roles in preventing phenylephrine (PE)-induced cardiac hypertrophy.
Methods and results
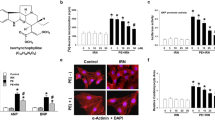
Immunocytochemistry (ICC) was employed for evaluating the morphology of the treated cardiomyocytes. Real-time PCR and western blot were respectively applied to assess the mRNA levels and protein expression of the relevant molecules. Bioinformatics analyses were carried out to investigate the potential mechanisms by which scutellarein modulated the PE-induced cardiac hypertrophy. The results showed that Scutellarein treatment significantly inhibited PE-induced increase in H9c2 and AC16 cardiomyocyte size. Besides, scutellarein treatment also dramatically suppressed the expression of the cardiac hypertrophic markers: ANP, BNP and β-MHC. Furthermore, the effects of scutellarein on attenuating the cardiac hypertrophy might be mediated by suppressing the activity of TRAF2/NF-κB signaling pathway.
Conclusions
Collectively, our data indicated that scutellarein could protect against PE-induced cardiac hypertrophy via regulating TRAF2/NF-κB signaling pathway using in vitro experiments.







Similar content being viewed by others
Data availability
The data and materials are available on reasonable request.
References
Gupta S, Das B, Sen S (2007) Cardiac hypertrophy: mechanisms and therapeutic opportunities. Antioxid Redox Signal 9:623–652. https://doi.org/10.1089/ars.2007.1474
Devereux RB, Roman MJ (1999) Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res 22:1–9. https://doi.org/10.1291/hypres.22.1
Xia Y, Karmazyn M (2004) Obligatory role for endogenous endothelin in mediating the hypertrophic effects of phenylephrine and angiotensin II in neonatal rat ventricular myocytes: evidence for two distinct mechanisms for endothelin regulation. J Pharmacol Exp Ther 310:43–51. https://doi.org/10.1124/jpet.104.065185
Frey N, Olson EN (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65:45–79. https://doi.org/10.1146/annurev.physiol.65.092101.142243
Bisping E, Wakula P, Poteser M, Heinzel FR (2014) Targeting cardiac hypertrophy: toward a causal heart failure therapy. J Cardiovasc Pharmacol 64:293–305. https://doi.org/10.1097/FJC.0000000000000126
Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR (2015) Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol 89:1401–1438. https://doi.org/10.1007/s00204-015-1477-x
Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Z (2001) The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol Cell Biol 21:3986–3994. https://doi.org/10.1128/MCB.21.12.3986-3994.2001
Liu SF, Malik AB (2006) NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290:L622–L645. https://doi.org/10.1152/ajplung.00477.2005
Kuusisto J, Karja V, Sipola P, Kholova I, Peuhkurinen K, Jaaskelainen P, Naukkarinen A, Yla-Herttuala S, Punnonen K, Laakso M (2012) Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 98:1007–1013. https://doi.org/10.1136/heartjnl-2011-300960
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071. https://doi.org/10.1056/NEJM199704103361506
Baeuerle PA, Baltimore D (1996) NF-kappa B: ten years after. Cell 87:13–20. https://doi.org/10.1016/s0092-8674(00)81318-5
Wang Z, Gao L, Xiao L, Kong L, Shi H, Tian X, Zhao L (2018) Bakuchiol protects against pathological cardiac hypertrophy by blocking NF-kappaB signaling pathway. Biosci Rep. https://doi.org/10.1042/BSR20181043
Manna SK, Babajan B, Raghavendra PB, Raviprakash N, Sureshkumar C (2010) Inhibiting TRAF2-mediated activation of NF-kappaB facilitates induction of AP-1. J Biol Chem 285:11617–11627. https://doi.org/10.1074/jbc.M109.094961
Su D, Cheng Y, Li S, Dai D, Zhang W, Lv M (2017) Sphk1 mediates neuroinflammation and neuronal injury via TRAF2/NF-kappaB pathways in activated microglia in cerebral ischemia reperfusion. J Neuroimmunol 305:35–41. https://doi.org/10.1016/j.jneuroim.2017.01.015
Gao R, Zhu BH, Tang SB, Wang JF, Ren J (2008) Scutellarein inhibits hypoxia- and moderately-high glucose-induced proliferation and VEGF expression in human retinal endothelial cells. Acta Pharmacol Sin 29:707–712. https://doi.org/10.1111/j.1745-7254.2008.00797.x
Li NG, Song SL, Shen MZ, Tang YP, Shi ZH, Tang H, Shi QP, Fu YF, Duan JA (2012) Mannich bases of scutellarein as thrombin-inhibitors: design, synthesis, biological activity and solubility. Bioorg Med Chem 20:6919–6923. https://doi.org/10.1016/j.bmc.2012.10.015
Sang Eun H, Seong Min K, Ho Jeong L, Vetrivel P, Venkatarame Gowda Saralamma V, Jeong Doo H, Eun Hee K, Sang Joon L, Sup K (2019) Scutellarein induces fas-mediated extrinsic apoptosis and G2/M cell cycle arrest in Hep3B hepatocellular carcinoma cells. Nutrients. https://doi.org/10.3390/nu11020263
Tang H, Tang Y, Li N, Shi Q, Guo J, Shang E, Duan JA (2014) Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats. Pharmacol Biochem Behav 118:51–59. https://doi.org/10.1016/j.pbb.2014.01.003
Zhou J, Lei H, Chen Y, Li F, Ma C (2002) Ventricular remodeling by Scutellarein treatment in spontaneously hypertensive rats. Chin Med J (Engl) 115:375–377
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566. https://doi.org/10.1056/NEJM199005313222203
Stoclet JC, Schini-Kerth V (2011) Dietary flavonoids and human health. Ann Pharm Fr 69:78–90. https://doi.org/10.1016/j.pharma.2010.11.004
Yan L, Huang H, Tang QZ, Zhu LH, Wang L, Liu C, Bian ZY, Li H (2010) Breviscapine protects against cardiac hypertrophy through blocking PKC-alpha-dependent signaling. J Cell Biochem 109:1158–1171. https://doi.org/10.1002/jcb.22495
Xing JF, You HS, Dong YL, Lu J, Chen SY, Zhu HF, Dong Q, Wang MY, Dong WH (2011) Metabolic and pharmacokinetic studies of scutellarin in rat plasma, urine, and feces. Acta Pharmacol Sin 32:655–663. https://doi.org/10.1038/aps.2011.11
You HS, Xing JF, Lu J, Dong WH, Liu JT, Dong YL (2014) Influence of the gastrointestinal microflora and efflux transporters on the absorption of scutellarin and scutellarein. Phytother Res 28:1295–1300. https://doi.org/10.1002/ptr.5127
Feng MQ, Song YH, Wu JX, Chen X, Bai XH, Zhang YQ (2017) Study on antitumour activity of scutellarin and its metabolite scutellarein by combining activity screening, target tissue distribution and pharmacokinetics. Chromatographia 80:427–435. https://doi.org/10.1007/s10337-017-3260-z
Bogoyevitch MA, Glennon PE, Sugden PH (1993) Endothelin-1, phorbol esters and phenylephrine stimulate MAP kinase activities in ventricular cardiomyocytes. FEBS Lett 317:271–275. https://doi.org/10.1016/0014-5793(93)81291-7
Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH (1998) The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J Biol Chem 273:7725–7730. https://doi.org/10.1074/jbc.273.13.7725
Valen G, Yan ZQ, Hansson GK (2001) Nuclear factor kappa-B and the heart. J Am Coll Cardiol 38:307–314. https://doi.org/10.1016/s0735-1097(01)01377-8
Saito T, Tanaka S (2017) Molecular mechanisms underlying osteoarthritis development: Notch and NF-kappaB. Arthritis Res Ther 19:94. https://doi.org/10.1186/s13075-017-1296-y
DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M (1997) A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548–554. https://doi.org/10.1038/41493
Baldwin AS Jr (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14:649–683. https://doi.org/10.1146/annurev.immunol.14.1.649
Thurberg BL, Collins T (1998) The nuclear factor-kappa B/inhibitor of kappa B autoregulatory system and atherosclerosis. Curr Opin Lipidol 9:387–396. https://doi.org/10.1097/00041433-199810000-00002
Etemadi N, Chopin M, Anderton H, Tanzer MC, Rickard JA, Abeysekera W, Hall C, Spall SK, Wang B, Xiong Y, Hla T, Pitson SM, Bonder CS, Wong WW, Ernst M, Smyth GK, Vaux DL, Nutt SL, Nachbur U, Silke J (2015) TRAF2 regulates TNF and NF-kappaB signalling to suppress apoptosis and skin inflammation independently of Sphingosine kinase 1. Elife. https://doi.org/10.7554/eLife.10592
Pomerantz JL, Baltimore D (1999) NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J 18:6694–6704. https://doi.org/10.1093/emboj/18.23.6694
Yamamoto S, Iwakuma T (2017) RIPK1-TRAF2 interplay on the TNF/NF-kappaB signaling, cell death, and cancer development in the liver. Transl Cancer Res 6:94–109. https://doi.org/10.21037/tcr.2017.04.01
Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW (1997) Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715–725. https://doi.org/10.1016/s1074-7613(00)80391-x
Huang Y, Wu D, Zhang X, Jiang M, Hu C, Lin J, Tang J, Wu L (2014) Cardiac-specific Traf2 overexpression enhances cardiac hypertrophy through activating AKT/GSK3beta signaling. Gene 536:225–231. https://doi.org/10.1016/j.gene.2013.12.052
Acknowledgements
This work was supported by the Science and Technology Commission of Shanghai Municipality (NO. 19DZ2201000) and the Outstanding Clinical Discipline Project of Shanghai Pudong (No.: PWYgy2018-10).
Funding
This work was supported by Science and Technology Commission of Shanghai Municipality (19DZ2201000) and the Outstanding Clinical Discipline Project of Shanghai Pudong (PWYgy2018-10).
Author information
Authors and Affiliations
Contributions
XS and JL conceived the study. XS, YH and YJ wrote the paper. XS, YH, YJ, JW, SW, YW, WD, CZ, JZ performed the experiments and the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Consent for publication
All authors agree to publish the study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, X., Hu, Y., Jiang, Y. et al. Scutellarein protects against cardiac hypertrophy via suppressing TRAF2/NF-κB signaling pathway. Mol Biol Rep 49, 2085–2095 (2022). https://doi.org/10.1007/s11033-021-07026-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-07026-0