Abstract
Background
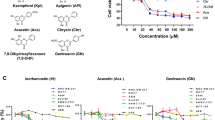
FMSP is a synthesized ferrocene derivative with anti-cancer characteristics on tumor cells. Naringenin is a polyphenolic flavonoid with anti-tumor ability.
Methods
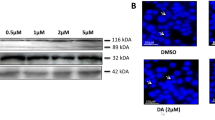
Cell viability and proliferation of two cancer cells and a normal cell line after treatment with these agents were determined with MTT assay. To predict the possible interaction between calmodulin (CaM) and FMSP and naringenin, docking studies were performed. By using fluorescence emission spectra, the effects of FMSP and naringenin on CaM structure and activity were studied. CaM-dependent activation of phosphodiesterase 1 (PDE1) by FMSP in relation to naringenin and their combination were compared. Effects of these compounds on PDE1 inhibition, cAMP accumulation, and cAMP-dependent protein kinase A (PKA) activation were assayed.
Results
The combination of FMSP and naringenin had more inhibitory effects on CaM structure than FMSP and naringenin alone. Results of docking analyses also confirmed efficient interaction of the two compounds with a hydrophobic pocket of calmodulin active site. Kinetic analyses of these agents’ interaction with CaM showed FMSP and naringenin both competitively inhibited PDE1 activation without changing the Vmax parameter. FMSP and naringenin synergistically increased Km values at a higher level compared to FMSP or naringenin alone. The combination of these two agents also had more cytotoxic effects on cancer cells than FMSP alone.
Conclusions
It was shown that mechanism of proliferation inhibition in both cancer cells by these compounds is based on CaM and consequent PDE inhibition followed by intracellular cAMP level elevation and increased PKA activity in a dose-dependent manner.






Similar content being viewed by others
Data availability
Our data is available upon any request from the corresponding author.
References
Sampaio MM, Santos MLC, Marques HS, Gonçalves VLS, Araújo GRL, Lopes LW, Apolonio JS, Silva CS, Santos LKS, Cuzzuol BR, Guimarães QES, Santos MN, de Brito BB, da Silva FAF, Oliveira MV, Souza CL, de Melo FF (2021) Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: a literature review. World J Clin Oncol 12:69–94. https://doi.org/10.5306/wjco.v12.i2.69
Sarno F, Pepe G, Termolino P, Carafa V, Massaro C, Merciai F, Campiglia P, Nebbioso A, Altucci L (2020) Trifolium repens blocks proliferation in chronic myelogenous leukemia via the BCR-ABL/STAT5 pathway. Cells 9:379. https://doi.org/10.3390/cells9020379
Kim E, Viatour P (2020) Hepatocellular carcinoma: old friends and new tricks. Experi Mol Med 52:1898–1907. https://doi.org/10.1038/s12276-020-00527-1
Jabbour E, Kantarjian H (2020) Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol 95(2020):691–709. https://doi.org/10.1002/ajh.25792
Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J (2019) Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer 9(2020):682–720. https://doi.org/10.1159/000509424
Nouri Z, Fakhri S, Nouri K, Wallace CE, Farzaei MH, Bishayee A (2020) Targeting multiple signaling pathways in cancer: the rutin therapeutic approach. Cancers (Basel) 12:2276. https://doi.org/10.3390/cancers12082276
Seebacher NA, Stacy AE, Porter GM, Merlot AM (2019) Clinical development of targeted and immune based anti-cancer therapies. J Exp Clin Cancer Res 38:156. https://doi.org/10.1186/s13046-019-1094-2
Soverini S, Mancini M, Bavaro L, Cavo M, Martinelli G (2018) Chronic myeloid leukemia: the paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy. Mol Cancer 17:49. https://doi.org/10.1186/s12943-018-0780-6
Roberts MJ, May LT, Keen AC, Liu B, Lam T, Charlton SJ, Rosethorne EM, Halls ML (2021) Inhibition of the proliferation of human lung fibroblasts by prostacyclin receptor agonists is linked to a sustained cAMP signal in the nucleus. Front Pharmacol 12:669227. https://doi.org/10.3389/fphar.2021.669227
Insel PA, Wilderman A, Zhang L, Keshwani MM, Zambon AC (2014) Cyclic AMP/PKA-promoted apoptosis: insights from studies of S49 lymphoma cells. Horm Metab Res 46:854–862. https://doi.org/10.1055/s-0034-1384519
Epstein PM (2017) Different phosphodiesterases (PDEs) regulate distinct phosphoproteomes during cAMP signaling. Proc Natl Acad Sci USA 114:7741–7743. https://doi.org/10.1073/pnas.1709073114
Omori K, Kotera J (2007) Overview of PDEs and their regulation. Circ Res 100:309–327. https://doi.org/10.1161/01.RES.0000256354.95791.f1
Shimizu K, Murata T, Watanabe Y, Sato C, Morita H, Tagawa T (2009) Characterization of phosphodiesterase 1 in human malignant melanoma cell lines. Anticancer Res 29:1119–1122
Yokokura S, Yurimoto S, Matsuoka A, Imataki O, Dobashi H, Bandoh S, Matsunaga T (2014) Calmodulin antagonists induce cell cycle arrest and apoptosis in vitro and inhibit tumor growth in vivo in human multiple myeloma. BMC Cancer 14:882. https://doi.org/10.1186/1471-2407-14-882
Noori S, Rajabi S, Tavirani MR, Shokri B, Zarghi A (2021) Anti-breast cancer activities of ketoprofen-RGD conjugate by targeting breast cancer stem-like cells and parental cells. Anticancer Agents Med Chem 21:1027–1036. https://doi.org/10.2174/1871520620666200908105416
Rajabi S, Shojaee M, Malmir A, Rezaei Tavirani M, Noori S (2020) Anti-breast cancer activities of 8-hydroxydaidzein by targeting breast cancer stem-like cellss. J Pharm Pharm Sci. 23:47–57
Nourbakhsh M, Farzaneh S, Taghikhani A, Zarghi A, Noori S (2020) The effect of a newly synthesized ferrocene derivative against MCF-7 breast cancer cells and spheroid stem cells through ros production and inhibition of JAK2/STAT3 signaling pathway. Anticancer Agents Med Chem 20:875–886. https://doi.org/10.2174/1871520620666200101151743
Lim W, Park S, Bazer FW, Song G (2017) Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J Cell Biochem 118:1118–1131. https://doi.org/10.1002/jcb.25729
Zhou J, Xia L, Zhang Y (2019) Naringin inhibits thyroid cancer cell proliferation and induces cell apoptosis through repressing PI3K/AKT pathway. Pathol Res Pract 215:152707. https://doi.org/10.1016/j.prp.2019.152707
Bao L, Liu F, Guo HB, Li Y, Tan BB, Zhang WX, Peng YH (2016) Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour Biol 37:11365–11374. https://doi.org/10.1007/s13277-016-5013-2
Farzaneh S, Zeinalzadeh E, Daraei B, Shahhosseini S, Zarghi A (2018) New ferrocene compounds as selective cyclooxygenase (COX-2) inhibitors: design, synthesis, cytotoxicity and enzyme-inhibitory activity. Anticancer Agents Med Chem 18:295–301. https://doi.org/10.2174/1871520617666171003145533
Pace CN (1990) Measuring and increasing protein stability. Trends Biotechnol 8(1990):93–98. https://doi.org/10.1016/0167-7799(90)90146-O
Villalobo A, Berchtold MW (2020) The role of calmodulin in tumor cell migration invasiveness, and metastasis. Int J Mol Sci 21:765. https://doi.org/10.3390/ijms21030765
Noori S, Hassan ZM (2014) Tehranolide inhibits cell proliferation via calmodulin inhibition, PDE, and PKA activation. Tumour Biol 35:257–264. https://doi.org/10.1007/s13277-013-1031-5
Hait WN, Morris S, Lazo JS, Figlin RJ, Durivage HJ, White K, Schwartz PE (1989) Phase I trial of combined therapy with bleomycin and the calmodulin antagonist, trifluoperazine. Cancer Chemother Pharmacol 23:358–362. https://doi.org/10.1007/bf00435836
Keravis T, Lugnier C (2012) Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol 165:1288–1305. https://doi.org/10.1111/j.1476-5381.2011.01729.x
Peng T, Gong J, Jin Y, Zhou Y, Tong R, Wei X, Bai L, Shi J (2018) Inhibitors of phosphodiesterase as cancer therapeutics. Eur J Med Chem 150:742–756. https://doi.org/10.1016/j.ejmech.2018.03.046
Zhao Z, Jin G, Ge Y, Guo Z (2019) Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology 27:1021–1036. https://doi.org/10.1007/s10787-018-00556-3
Park HJ, Choi YJ, Lee JH, Nam MJ (2017) Naringenin causes ASK1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food Chem Toxicol 99:1–8. https://doi.org/10.1016/j.fct.2016.11.008
Acknowledgements
The authors thank the Vice Chancellor for Research of Shahid Beheshti University of Medical Sciences.
Funding
No funding resources were received.
Author information
Authors and Affiliations
Contributions
SN and AZ designed the experiment and research methodology. MAM and SF performed the experiments, while SR and MRA were involved in the data analysis and manuscript write-up.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajabi, S., Noori, S., Ashrafi, M.R. et al. Naringenin enhances anti-proliferation effect of 1-ferrocenyl-3-(4-methylsulfonylphenyl) propen-1-one on two different cells via targeting calmodulin signaling pathway. Mol Biol Rep 49, 1027–1036 (2022). https://doi.org/10.1007/s11033-021-06923-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06923-8