Abstract
Background
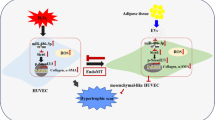
Diabetic nephropathy (DN), is microvascular complication of diabetes causes to kidney dysfunction and renal fibrosis. It is known that hyperglycemia and advanced glycation end products (AGEs) produced by hyperglycemic condition induce myofibroblast differentiation and endothelial-to-mesenchymal transition (EndoMT), and exacerbate fibrosis in DN. Recently, we demonstrated that α2-antiplasmin (α2AP) is associated with inflammatory response and fibrosis progression.
Methods
We investigated the role of α2AP on fibrosis progression in DN using a streptozotocin-induced DN mouse model.
Results
α2AP deficiency attenuated EndoMT and fibrosis progression in DN model mice. We also showed that the high glucose condition/AGEs induced α2AP production in fibroblasts (FBs), and the reduction of receptor for AGEs (RAGE) by siRNA attenuated the AGEs-induced α2AP production in FBs. Furthermore, the bloackade of α2AP by the neutralizing antibody attenuated the high glucose condition-induced pro-fibrotic changes in FBs. On the other hand, the hyperglycemic condition/AGEs induced EndoMT in vascular endothelial cells (ECs), the FBs/ECs co-culture promoted the high glucose condition-induced EndoMT compared to ECs mono-culture. Furthermore, α2AP promoted the AGEs-induced EndoMT, and the blockade of α2AP attenuated the FBs/ECs co-culture-promoted EndoMT under the high glucose condition.
Conclusions
The high glucose conditions induced α2AP production, and α2AP is associated with EndoMT and fibrosis progression in DN. These findings provide a basis for clinical strategies to improve DN.






Similar content being viewed by others
References
Kanasaki K, Taduri G, Koya D (2013) Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne) 4:7
LeBleu V, Taduri G, O’Connell J, Teng Y, Cooke V, Woda C, Sugimoto H, Kalluri R (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19:1047–1053
Srivastava S, Koya D, Kanasaki K (2013) MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT and EndMT. Biomed Res Int 2013:125469
Kanno Y (2019) The role of fibrinolytic regulators in vascular dysfunction of systemic sclerosis. Int J Mol Sci 20:E619
Li J, Qu X, Yao J, Caruana G, Ricardo S, Yamamoto Y, Yamamoto H, Bertram J (2010) Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 59:2612–2624
Sanajou D, Ghorbani Haghjo A, Argani H, Aslani S (2018) AGE-RAGE axis blockade in diabetic nephropathy: current status and future directions. Eur J Pharmacol 833:158–164
Kumar Pasupulati A, Chitra P, Reddy G (2016) Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol Concepts 7:293–309
Kanno Y, Ishisaki A, Kawashita E, Kuretake H, Ikeda K, Matsuo O (2016) uPA attenuated LPS-induced inflammatory osteoclastogenesis through the plasmin/PAR-1/Ca2+/CaMKK/AMPK axis. Int J Biol Sci 12:63–71
Kanno Y, Ishisaki A, Kawashita E, Chosa N, Nakajima K, Nishihara T, Toyoshima K, Okada K, Ueshima S, Matsushita K, Matsuo O, Matsuno H (2011) Plasminogen/plasmin modulates bone metabolism by regulating the osteoblast and osteoclast function. J Biol Chem 286:8952–8960
Menoud P, Sappino N, Boudal-Khoshbeen M, Vassalli J, Sappino A (1996) The kidney is a major site of alpha2-antiplasmin production. J Clin Invest 97:2478–2484
Kanno Y, Hirade K, Ishisaki A, Nakajima K, Suga H, Into T, Matsushita K, Okada K, Matsuo O, Matsuno H (2006) Lack of alpha2-antiplasmin improves cutaneous wound healing via over-released vascular endothelial growth factor-induced angiogenesis in wound lesions. J Thromb Haemost 4:1602–1610
Kanno Y, Miyashita M, Seishima M, Matsuo O (2020) α2AP is associated with the development of lupus nephritis through the regulation of plasmin inhibition and inflammatory responses. Immun Inflamm Dis 8:267–278
Kanno Y, Ishisaki A, Kuretake H, Maruyama C, Matsuda A, Matsuo O (2017) alpha2-antiplasmin modulates bone formation by negatively regulating osteoblast differentiation and function. Int J Mol Med 40:854–858
Kanno Y, Kawashita E, Minamida M, Kaneiwa A, Okada K, Ueshima S, Matsuo O, Matsuno H (2010) alpha2-antiplasmin is associated with the progression of fibrosis. Am J Pathol 176:238–245
Kanno Y, Kawashita E, Kokado A, Kuretake H, Ikeda K, Okada K, Seishima M, Ueshima S, Matsuo O, Matsuno H (2014) α2AP mediated myofibroblast formation and the development of renal fibrosis in unilateral ureteral obstruction. Sci Rep 4:5967
Kanno Y, Shu E, Kanoh H, Seishima M (2016) The antifibrotic effect of α2AP neutralization in systemic sclerosis dermal fibroblasts and mouse models of systemic sclerosis. J Invest Dermatol 136:762–769
Kanno Y, Kawashita E, Kokado A, Okada K, Ueshima S, Matsuo O, Matsuno H (2013) Alpha2-antiplasmin regulates the development of dermal fibrosis in mice by prostaglandin F2α synthesis through adipose triglyceride lipase/calcium-independent phospholipase A2. Arthritis Rheum 65:492–502
Kanno Y, Shu E, Kanoh H, Matsuda A, Seishima M (2017) α2AP regulates vascular alteration by inhibiting VEGF signaling in systemic sclerosis: the roles of α2AP in vascular dysfunction in systemic sclerosis. Arthritis Res Ther 19:22
Polat S, Ugurlu N, Yulek F, Simavli H, Ersoy R, Cakir B, Erel O (2014) Evaluation of serum fibrinogen, plasminogen, α2-anti-plasmin, and plasminogen activator inhibitor levels (PAI) and their correlation with presence of retinopathy in patients with type 1 DM. J Diabetes Res 2014:317292
Aso Y, Fujiwara Y, Tayama K, Takebayashi K, Inukai T, Takemura Y (2000) Relationship between soluble thrombomodulin in plasma and coagulation or fibrinolysis in type 2 diabetes. Clin Chim Acta 301:135–145
Okada K, Lijnen H, Dewerchin M, Belayew A, Matsuo O, Collen D, Bernaerts R (1997) Characterization and targeting of the murine alpha2-antiplasmin gene. Thromb Haemost 78:1104–1110
Kanno Y, Kaneiwa A, Minamida M, Kanno M, Tomogane K, Takeuchi K, Okada K, Ueshima S, Matsuo O, Matsuno H (2008) The absence of uPAR is associated with the progression of dermal fibrosis. J Invest Dermatol 128:2792–2797
Kanno Y, Into T, Lowenstein C, Matsushita K (2008) Nitric oxide regulates vascular calcification by interfering with TGF-b signalling. Cardiovasc Res 77:221–230
Kanno Y, Ishisaki A, Miyashita M, Matsuo O (2016) The blocking of uPAR suppresses lipopolysaccharide-induced inflammatory osteoclastogenesis and the resultant bone loss through attenuation of integrin β3/Akt pathway. Immun Inflamm Dis 4:338–349
Kanno Y, Maruyama C, Matsuda A, Ishisaki A (2017) uPA-derived peptide, Å6 is involved in the suppression of lipopolysaccaride-promoted inflammatory osteoclastogenesis and the resultant bone loss. Immun Inflamm Dis 5:289–299
Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV (2012) A multimodal RAGE-specific inhibitor reduces amyloid b-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 122:1377–1392
Xu J, Tao B, Guo X, Zhou S, Li Y, Zhang Y, Zhou Z, Cheng H, Zhang X, Ke Y (2017) Macrophage-restricted Shp2 tyrosine phosphatase acts as a rheostat for MMP12 through TGF-b activation in the prevention of age-related emphysema in mice. J Immunol 199:2323–2332
Han Y, Xu X, Tang C, Gao P, Chen X, Xiong X (2018) Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial rostxnip-nlrp3 biological axis. Redox Biol 16:32–46
Srivastava S, Shi S, Koya D, Kanasaki K (2014) Lipid mediators in diabetic nephropathy. Fibrogenesis Tissue Repair 7:12
Kawashita E, Kanno Y, Asayama H, Okada K, Ueshima S, Matsuo O, Matsuno H (2013) Involvement of α2-antiplasmin in dendritic growth of hippocampal neurons. J Neurochem 126:58–69
Erem C, Hacihasanoğlu A, Celik S, Ovali E, Ersöz H, Ukinç K, Deger O, Telatar M (2005) Coagulation and fibrinolysis parameters in type 2 diabetic patients with and without diabetic vascular complications. Med Princ Pract 14:22–30
Madan R, Gupt B, Saluja S, Kansra U, Tripathi B, Guliani B (2010) Coagulation profile in diabetes and its association with diabetic microvascular complications. J Assoc Phys India 58:481–484
Waasdorp M, Duitman J, Spek C (2017) Plasmin reduces fibronectin deposition by mesangial cells in a protease-activated receptor-1 independent manner. Biochem Biophys Rep 10:152–156
Zhou X, Muise E, Haimbach R, Sebhat I, Zhu Y, Liu F, Souza S, Kan Y, Pinto S, Kelley D, Hoek M (2019) PAN-AMPK activation improves renal function in a rat model of progressive diabetic nephropathy. J Pharmacol Exp Ther 371:45–55
Author information
Authors and Affiliations
Contributions
YK conceived and designed the experiment. YK, MH and OM were involved in the mice experiments. YK and MH analyzed the data. YK, OM and KO were involved in data interpretation and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The mice experiments in this study were approved by the Animal Research Committee of Doshisha Women’s College of Liberal Arts (Approval ID: Y17-015), and were carried out in accordance with the rules and regulations of the institutions and the government.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kanno, Y., Hirota, M., Matsuo, O. et al. α2-antiplasmin positively regulates endothelial-to-mesenchymal transition and fibrosis progression in diabetic nephropathy. Mol Biol Rep 49, 205–215 (2022). https://doi.org/10.1007/s11033-021-06859-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06859-z