Abstract
Background
Obesity is considered a chronic inflammatory disease and transforming growth factor beta 1 (TGFβ1) might exert important roles in disease pathogenesis regulating adipocyte differentiation and immune-inflammatory environment. However, the role of this cytokine as a biomarker in obesity is poorly addressed. Therefore, the present study aimed to evaluate the impact of TGFB1 polymorphisms and TGFβ1 plasmatic levels in obesity
Methods and results
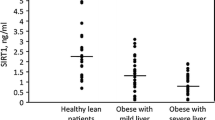
TGFB1 promoter region polymorphisms (rs1800468, G-800A and rs1800469, C-509 T) were evaluated in 75 obese patients and 45 eutrophic patients through PCR–RFLP and plasmatic TGFβ1 was quantified through ELISA from 37 of the obese patients, and correlations with clinical and biochemical parameters were tested. Despite no association was found between TGFB1 polymorphisms and obesity susceptibility, several correlations with clinical data were noted. Among others, AC haplotype negatively correlated with plasmatic TGFβ1, while plasmatic TGFβ1 negatively correlated with C-reactive protein and positively correlated with liver abnormalities on ultrasound and, specifically, with steatosis presence and degree. Conversely, GT haplotype, which associates with higher TGFβ1 production, was also positively correlated with the same parameters of liver abnormalities. Further, plasmatic vitamin D negatively correlated with TGFβ1, while positively correlated with AC haplotype.
Conclusion
Overall, the results indicate that TGFβ1 might exert important roles in obesity pathophysiology and correlate with biochemical and clinical parameters both at systemic protein as well as at genetic level. Importantly, the consistent positive correlation at both levels with steatosis might suggest this cytokine as a biomarker for this hepatic abnormality in obese patients.

Similar content being viewed by others
Data availability
Data are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Mohammed MS, Sendra S, Lloret J, Bosch I (2018) Systems and WBANs for controlling obesity. J Healthc Eng 2018:1564748. https://doi.org/10.1155/2018/1564748
Organization WH (2014) Global Health Observatory. Obesity: Situation and trends. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
de Risco VVdF (2014) e Proteçao para Doenças Crônicas por Inquérito Telefônico (2013). Ministério da Saúde Brasil Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2015.pdf
Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y (2017) Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 13(4):851–863. https://doi.org/10.5114/aoms.2016.58928
Gregor MF, Hotamisligil GS (2011) Inflammatory Mechanisms in Obesity. Annu Rev Immunol 29(1):415–445. https://doi.org/10.1146/annurev-immunol-031210-101322
Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S (2012) TGF-beta - an excellent servant but a bad master. J Transl Med 10:183. https://doi.org/10.1186/1479-5876-10-183
Ciardiello D, Elez E, Tabernero J, Seoane J (2020) Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann Oncol 31(10):1336–1349. https://doi.org/10.1016/j.annonc.2020.07.009
Roberts AB (1998) Molecular and cell biology of TGF-β. Miner Electrolyte Metab 24(2–3):111–119. https://doi.org/10.1159/000057358
Batlle E, Massagué J (2019) Transforming growth factor-β signaling in immunity and cancer. Immunity 50(4):924–940. https://doi.org/10.1016/j.immuni.2019.03.024
Sanjabi S, Oh SA, Li MO (2017) Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol 9(6):a022236. https://doi.org/10.1101/cshperspect.a022236
Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D (2009) Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15(8):930–939. https://doi.org/10.1038/nm.2002
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Investig 117(1):175–184. https://doi.org/10.1172/jci29881
Pereira S, Teixeira L, Aguilar E, Oliveira M, Savassi-Rocha A, Pelaez JN, Capettini L, Diniz MT, Ferreira A, Alvarez-Leite J (2014) Modulation of adipose tissue inflammation by FOXP3+ Treg cells, IL-10, and TGF-β in metabolically healthy class III obese individuals. Nutrition 30(7–8):784–790. https://doi.org/10.1016/j.nut.2013.11.023
Petruschke T, Röhrig K, Hauner H (1994) Transforming growth factor beta (TGF-beta) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int J Obes Relat Metab Disord 18(8):532–536
Lee M-J (2018) Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim et Biophys Acta–Mol Basis Dis 1864(4):1160–1171. https://doi.org/10.1016/j.bbadis.2018.01.025
Choy L, Skillington J, Derynck R (2000) Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol 149(3):667–682. https://doi.org/10.1083/jcb.149.3.667
Yadav H, Quijano C, Kamaraju Anil K, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright Elizabeth C, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner Anne E, Finkel T, Rane Sushil G (2011) Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 14(1):67–79. https://doi.org/10.1016/j.cmet.2011.04.013
Reggio S, Rouault C, Poitou C, Bichet J-C, Prifti E, Bouillot J-L, Rizkalla S, Lacasa D, Tordjman J, Clément K (2016) Increased basement membrane components in adipose tissue during obesity: links with TGFβ and metabolic phenotypes. J Clin Endocrinol Metab 101(6):2578–2587. https://doi.org/10.1210/jc.2015-4304
Gagnon AM, Chabot J, Pardasani D, Sorisky A (1998) Extracellular matrix induced by TGFβ impairs insulin signal transduction in 3T3-L1 preadipose cells. J Cell Physiol 175(3):370–378. https://doi.org/10.1002/(sici)1097-4652(199806)175:3%3c370::aid-jcp15%3e3.0.co;2-9
Grainger DJ, Heathcote K, Chiano M, Snieder H, Kemp PR, Metcalfe JC, Carter ND, Spector TD (1999) Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet 8(1):93–97
Martelossi Cebinelli GC, Paiva Trugilo K, Badaró Garcia S, Brajão de Oliveira K (2016) TGF-β1 functional polymorphisms: a review. Eur Cytokine Netw 27(4):81–89. https://doi.org/10.1684/ecn.2016.0382
Vitiello GAF, Amarante MK, Oda JMM, Hirata BKB, de Oliveira CEC, Campos CZ, de Oliveira KB, Guembarovski RL, Watanabe MAE (2020) Transforming growth factor beta 1 (TGFβ1) plasmatic levels in breast cancer and neoplasia-free women: Association with patients’ characteristics and TGFB1 haplotypes. Cytokine 130:155079. https://doi.org/10.1016/j.cyto.2020.155079
Cotton SA, Gbadegesin RA, Williams S, Brenchley PEC, Webb NJA (2002) Role of TGF-β1 in renal parenchymal scarring following childhood urinary tract infection. Kidney Int 61(1):61–67. https://doi.org/10.1046/j.1523-1755.2002.00110.x
Alessi M-C, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I (2000) Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 49(8):1374–1380
Rosmond R, Chagnon M, Bouchard C, Björntorp P (2003) Increased abdominal obesity, insulin and glucose levels in nondiabetic subjects with a T29C polymorphism of the transforming growth factor-beta1 gene. Horm Res 59(4):191–194. https://doi.org/10.1159/000069323
Keophiphath M, Achard V, Henegar C, Rouault C, Clément K, Lacasa D (2009) Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol 23(1):11–24. https://doi.org/10.1210/me.2008-0183
Bourlier V, Sengenès C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, Villageois P, Esteve D, Chiotasso P, Dani C, Bouloumié A (2012) TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS ONE 7(2):e31274. https://doi.org/10.1371/journal.pone.0031274
Ramos MRZ, de Oliveira CL, Wagner NRF, Felicidade I, da Cruz MR, Taconeli CA, Fernandes R, Filho AJB, Campos ACL (2021) Effects of Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 supplementation on nutritional and metabolic parameters in the early postoperative period after Roux-en-Y gastric bypass: a Randomized, double-blind. Placebo-Controlled Trial Obes Surg 31(5):2105–2114. https://doi.org/10.1007/s11695-021-05222-2
Jin Q, Hemminki K, Grzybowska E, Klaes R, Söderberg M, Zientek H, Rogozinska-Szczepka J, Utracka-Hutka B, Pamula J, Pekala W, Försti A (2004) Polymorphisms and haplotype structures in genes for transforming growth factor beta1 and its receptors in familial and unselected breast cancers. Int J Cancer 112(1):94–99. https://doi.org/10.1002/ijc.20370
Vitiello GAF, Guembarovski RL, Hirata BKB, Amarante MK, de Oliveira CEC, de Oliveira KB, Cebinelli GCM, Guembarovski AL, Campos CZ, Watanabe MAE (2018) Transforming growth factor beta 1 (TGFβ1) polymorphisms and haplotype structures have dual roles in breast cancer pathogenesis. J Cancer Res Clin Oncol 144(4):645–655. https://doi.org/10.1007/s00432-018-2585-9
Stephens M, Scheet P (2005) Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genetics 76(3):449–462. https://doi.org/10.1086/428594
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genetics 68(4):978–989. https://doi.org/10.1086/319501
Jung UJ, Choi MS (2014) Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 15(4):6184–6223. https://doi.org/10.3390/ijms15046184
Lee BC (1842) Lee J (2014) Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochem Biophys Acta 3:446–462. https://doi.org/10.1016/j.bbadis.2013.05.017
Samad F, Pandey M, Loskutoff DJ (1998) Tissue factor gene expression in the adipose tissues of obese mice. Proc Natl Acad Sci USA 95(13):7591–7596. https://doi.org/10.1073/pnas.95.13.7591
Samad F, Uysal KT, Wiesbrock SM, Pandey M, Hotamisligil GS, Loskutoff DJ (1999) Tumor necrosis factor is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc Natl Acad Sci 96(12):6902–6907. https://doi.org/10.1073/pnas.96.12.6902
Sparks RL, Allen BJ, Zygmunt AI, Strauss EE (1993) Loss of differentiation control in transformed 3T3 T proadipocytes. Cancer Res 53(8):1770–1776
Bortell R, Owen TA, Ignotz R, Stein GS, Stein JL (1994) TGF beta 1 prevents the down-regulation of type I procollagen, fibronectin, and TGF beta 1 gene expression associated with 3T3-L1 pre-adipocyte differentiation. J Cell Biochem 54(2):256–263. https://doi.org/10.1002/jcb.240540214
Han H, Chung SI, Park HJ, Oh EY, Kim S-R, Park KH, Lee J-H, Park J-W (2021) Obesity-induced vitamin D deficiency contributes to lung fibrosis and airway hyperresponsiveness. Am J Respir Cell Mol Biol 64(3):357–367. https://doi.org/10.1165/rcmb.2020-0086OC
Barretto JR, Boa-Sorte N, Vinhaes CL, Malta-Santos H, Rebouças-Silva J, Ramos CF, Torres-Nascimento MAS, Borges VM, Andrade BB (2020) Heightened plasma levels of transforming growth factor beta (TGF-β) and increased degree of systemic biochemical perturbation characterizes hepatic steatosis in overweight pediatric patients: a cross-sectional study. Nutrients 12(6):1650. https://doi.org/10.3390/nu12061650
Yoshimoto N, Togo S, Kubota T, Kamimukai N, Saito S, Nagano Y, Endo I, Sekido H, Nagashima Y, Shimada H (2005) Role of transforming growth factor-beta1 (TGF-beta1) in endotoxin-induced hepatic failure after extensive hepatectomy in rats. J Endotoxin Res 11(1):33–39. https://doi.org/10.1179/096805105225006650
Yu X, Guo R, Ming D, Deng Y, Su M, Lin C, Li J, Lin Z, Su Z (2015) The transforming growth factor β1/Interleukin-31 pathway is upregulated in patients with hepatitis B virus-related acute-on-chronic liver failure and is associated with disease severity and survival. Clin Vaccine Immunol 22(5):484–492. https://doi.org/10.1128/cvi.00649-14
Nair B, Nath LR (2020) Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J Recept Signal Transduct 40(3):195–200. https://doi.org/10.1080/10799893.2020.1726952
Silverman ES, Palmer LJ, Subramaniam V, Hallock A, Mathew S, Vallone J, Faffe DS, Shikanai T, Raby BA, Weiss ST, Shore SA (2004) Transforming growth factor-beta1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med 169(2):214–219. https://doi.org/10.1164/rccm.200307-973OC
Dixon JB, Bhathal PS, Jonsson JR, Dixon AF, Powell EE, O’Brien PE (2003) Pro-fibrotic polymorphisms predictive of advanced liver fibrosis in the severely obese. J Hepatol 39(6):967–971. https://doi.org/10.1016/s0168-8278(03)00459-8
Sánchez-Parada MG, Alvarez-Rodríguez BA, Gómez-Meda BC, Troyo-Sanromán R, Sánchez-Orozco LV, Zamora-Perez AL, Landeros MSL, Armendáriz-Borunda J (2015) Association of genetic polymorphisms with histological grading of necroinflammation, staging of fibrosis, and liver function in Mexicans with chronic hepatitis C virus infection. J Investig Med 61(7):1088–1096. https://doi.org/10.2310/JIM.0b013e3182a32e24
Bader El Din NG, Farouk S, El-Shenawy R, Elhady MM, Ibrahim MK, Dawood RM, Salem AM, El Awady MK (2017) The synergistic effect of TNFα -308 G/A and TGFβ1 -509 C/T polymorphisms on hepatic fibrosis progression in hepatitis C virus genotype 4 patients. Viral Immunol 30(2):127–135. https://doi.org/10.1089/vim.2016.0083
Fink LN, Oberbach A, Costford SR, Chan KL, Sams A, Blüher M, Klip A (2013) Expression of anti-inflammatory macrophage genes within skeletal muscle correlates with insulin sensitivity in human obesity and type 2 diabetes. Diabetologia 56(7):1623–1628. https://doi.org/10.1007/s00125-013-2897-x
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72(3):690–693. https://doi.org/10.1093/ajcn/72.3.690
Pramyothin P, Biancuzzo RM, Lu Z, Hess DT, Apovian CM, Holick MF (2011) Vitamin D in adipose tissue and serum 25-hydroxyvitamin D after Roux-en-Y gastric bypass. Obesity 19(11):2228–2234. https://doi.org/10.1038/oby.2011.170
Drincic A, Fuller E, Heaney RP, Armas LAG (2013) 25-Hydroxyvitamin D response to graded vitamin D3supplementation among obese adults. J Clin Endocrinol Metab 98(12):4845–4851. https://doi.org/10.1210/jc.2012-4103
Minelli C, Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, Wood AR, Michaëlsson K, Vandenput L, Zgaga L, Yerges-Armstrong LM, McCarthy MI, Dupuis J, Kaakinen M, Kleber ME, Jameson K, Arden N, Raitakari O, Viikari J, Lohman KK, Ferrucci L, Melhus H, Ingelsson E, Byberg L, Lind L, Lorentzon M, Salomaa V, Campbell H, Dunlop M, Mitchell BD, Herzig K-H, Pouta A, Hartikainen A-L, Streeten EA, Theodoratou E, Jula A, Wareham NJ, Ohlsson C, Frayling TM, Kritchevsky SB, Spector TD, Richards JB, Lehtimäki T, Ouwehand WH, Kraft P, Cooper C, März W, Power C, Loos RJF, Wang TJ, Järvelin M-R, Whittaker JC, Hingorani AD, Hyppönen E (2013) Causal relationship between obesity and vitamin D status: bi-directional mendelian randomization analysis of multiple cohorts. PLoS Med 10(2):e1001383. https://doi.org/10.1371/journal.pmed.1001383
Wang Z, Zhang H, Sun X, Ren L (2016) The protective role of vitamin D3 in a murine model of asthma via the suppression of TGF-β/Smad signaling and activation of the Nrf2/HO-1 pathway. Mol Med Rep 14(3):2389–2396. https://doi.org/10.3892/mmr.2016.5563
Li S-R, Tan Z-X, Chen Y-H, Hu B, Zhang C, Wang H, Zhao H, Xu D-X (2019) Vitamin D deficiency exacerbates bleomycin-induced pulmonary fibrosis partially through aggravating TGF-β/Smad2/3-mediated epithelial-mesenchymal transition. Respir Res. https://doi.org/10.1186/s12931-019-1232-6
Ito I, Waku T, Aoki M, Abe R, Nagai Y, Watanabe T, Nakajima Y, Ohkido I, Yokoyama K, Miyachi H, Shimizu T, Murayama A, Kishimoto H, Nagasawa K, Yanagisawa J (2013) A nonclassical vitamin D receptor pathway suppresses renal fibrosis. J Clin Investig 123(11):4579–4594. https://doi.org/10.1172/jci67804
Jiang F, Yang Y, Xue L, Li B, Zhang Z (2017) 1α,25-Dihydroxyvitamin D3 attenuates TGF-β-induced pro-fibrotic effects in human lung epithelial cells through inhibition of epithelial-mesenchymal transition. Nutrients 9(9):980. https://doi.org/10.3390/nu9090980
Zerr P, Vollath S, Palumbo-Zerr K, Tomcik M, Huang J, Distler A, Beyer C, Dees C, Gela K, Distler O, Schett G, Distler JHW (2015) Vitamin D receptor regulates TGF-β signalling in systemic sclerosis. Ann Rheum Dis 74(3):e20–e20. https://doi.org/10.1136/annrheumdis-2013-204378
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15(5):288–298. https://doi.org/10.1038/s41574-019-0176-8
Endalifer ML, Diress G (2020) Epidemiology, predisposing factors, biomarkers, and prevention mechanism of obesity: a systematic review. J Obes 2020:1–8. https://doi.org/10.1155/2020/6134362
Oussaada SM, van Galen KA, Cooiman MI, Kleinendorst L, Hazebroek EJ, van Haelst MM, ter Horst KW, Serlie MJ (2019) The pathogenesis of obesity. Metabolism 92:26–36. https://doi.org/10.1016/j.metabol.2018.12.012
Bolton J, Montastier E, Carayol J, Bonnel S, Mir L, Marques MA, Astrup A, Saris W, Iacovoni J, Villa-Vialaneix N, Valsesia A, Langin D, Viguerie N (2017) Molecular biomarkers for weight control in obese individuals subjected to a multiphase dietary intervention. J Clin Endocrinol Metab 102(8):2751–2761. https://doi.org/10.1210/jc.2016-3997
Chielle EO et al (2018) Influence of obesity and overweight on transforming growth factor beta 1 levels and other oxidative and cardiometabolic parameters. Clin Biomed Res 38(3). Accessed 14 Aug 2021
Tan CK, Chong HC, Tan EH, Tan NS (2012) Getting “Smad” about obesity and diabetes. Nutr Diabetes 2(3):e29. https://doi.org/10.1038/nutd.2012.1
Acknowledgements
The authors acknowledge all the volunteer donors involved in this study from Blood Center of the Hospital das Clínicas/UNESP—School of Medicine in Botucatu, State University of São Paulo (São Paulo, Brazil) and staff from Hospital Santa Casa de Misericórdia in Curitiba (Paraná, Brazil) for the support during sample collection. This study was supported by CNPq (Proc. n° 152170/2019-7 and n° 306386/2017-8), CAPES, and FINEP.
Funding
This study was supported by the Brazilian National Council for Scientific and Technological Development (CNPq, Processes n° 152170/2019-7 and n° 306386/2017-8), the Coordination of Superior Level Staff Improvement (CAPES), and the Brazilian Innovation Agency (FINEP).
Author information
Authors and Affiliations
Contributions
IF: conceptualization, methodology, investigation, formal analysis, data curation, writing—original draft. MB: methodology, investigation, formal analysis, writing—original draft. MRZR: conceptualization, data curation, supervision, project administration, writing—original draft. LOC: data curation, formal analysis. NRFW: data curation, formal analysis. ACLC: resources, supervision. LRR: supervision, funding acquisition, resources, project administration. MSM: supervision, funding acquisition, resources, project administration. MAEW: supervision, resources, project administration, writing—review & editing. GAFV: conceptualization, methodology, formal analysis, visualization, data curation, supervision, writing—original draft, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
The study protocol was submitted and approved (Protocol No. 3799/2011 and CAAE 79894217.9.0000.0020) by the Human Research Ethics Committee of the UNESP School of Medicine (Botucatu, São Paulo, Brazil) and by the Pontifical Catholic University of Paraná (PUCPR—Curitiba, Paraná, Brazil), respectively, according to Resolution No. 466/12 of the Brazilian National Health Council.
Consent to participate
Signed free-informed consent was obtained from all individual participants included in the study prior to biological material or clinical data collection.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Felicidade, I., Bocchi, M., Ramos, M.R.Z. et al. Transforming growth factor beta 1 (TGFβ1) plasmatic levels and haplotype structures in obesity: a role for TGFβ1 in steatosis development. Mol Biol Rep 48, 6401–6411 (2021). https://doi.org/10.1007/s11033-021-06640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06640-2