Abstract
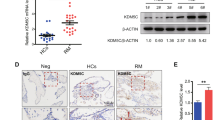
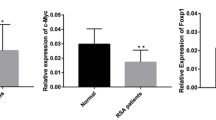
Some studies suggest that the inactivation of the Ras-MAPK pathway in trophoblast cells can lead to recurrent abortion, but the molecular mechanism underlying the inactivation of this pathway in trophoblast cells is still unclear. This study aimed to explore the relationship between the mechanism of abnormal activation of RASA1, a regulatory protein of the Ras-MAPK pathway, and unexplained recurrent spontaneous abortion. RT-qPCR was used to detect the transcription levels of RASA1 gene. Immunohistochemistry and Western blot were used to detect the expression levels of the RASA1, Raf and MEK proteins. CCK-8, TUNEL and Transwell assays were used to detect the proliferative, apoptotic, and invasive capacities of HTR-8/SVneo cells. ChIP assays were used to detect the enrichment of H3K27me3 in RASA1 gene promoter. Abortion villi experiments showed that the enrichment of H3K27me3 in the RASA1 gene promoter was reduced, and that both RASA1 gene transcription and RASA1 protein expression were increased. Cell experiments confirmed that RASA1 could decrease the phosphorylated Raf and MEK proteins, inhibit the proliferation and invasion ability, and promote the apoptosis ability of HTR-8/SVneo cells. It was also found that the proliferation and invasion ability as well as the Ras-MAPK pathway activity of HTR-8/SVneo cells were inhibited when treated with histone methyltransferase inhibitor DZNep. RASA1 gene was abnormally activated in unexplained recurrent spontaneous abortion villi due to the decreased enrichment of H3K27me3 in the gene promoter. High expression of RASA1 could inhibit the activity of the Ras-MAPK pathway, and thus inhibit the proliferation and invasion ability of trophoblast cells.





Similar content being viewed by others
Data Availability
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Abbreviations
- ChIP:
-
Chromatin immunoprecipitation
- lncRNA:
-
Long noncoding RNA
- MEK:
-
Mitogen-activated protein kinase kinase
- Raf:
-
Rapidly accelerated fibrosarcoma
- RASA1:
-
RAS P21 Protein Activator 1
- shRNA:
-
Short hairpin RNA
- URSA:
-
Unexplained recurrent spontaneous abortion
References
Ewington LJ, Tewary S, Brosens JJ (2019) New insights into the mechanisms underlying recurrent pregnancy loss. J Obstet Gynaecol Res 45:258–265
Meng L, Lin J, Chen L, Wang Z, Liu M, Liu Y, Chen X, Zhu L, Chen H, Zhang J (2016) Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch Gynecol Obstet 294:29–39
Liu HN, Tang XM, Wang XQ, Gao J, Li N, Wang YY, Xia HF (2020) MiR-93 Inhibits Trophoblast cell proliferation and promotes cell apoptosis by targeting BCL2L2 in recurrent spontaneous abortion. Reprod Sci 27:152–162
Wu L, Cheng B, Liu Q, Jiang P, Yang J (2020) CRY2 suppresses trophoblast migration and invasion in recurrent spontaneous abortion. J Biochem 167:79–87
Luan X, Li S, Zhao J, Zhai J, Liu X, Chen ZJ, Li W, Du Y (2020) Down-regulation of CCR7 via AKT pathway and GATA2 inactivation suppressed trophoblast migration and invasion in recurrent spontaneous abortion. Biol Reprod 102:424–433
Xiao Q, Zeng FL, Tang GY, Lei CY, Zou XX, Liu XL, Peng BL, Qin S, Li HX (2019) Expression of galectin-3 and apoptosis in placental villi from patients with missed abortion during early pregnancy. Exp Ther Med 17:2623–2631
Moser G, Windsperger K, Pollheimer J, de Sousa Lopes SC, Huppertz B (2018) Human trophoblast invasion: new and unexpected routes and functions. Histochem Cell Biol 150:361–370
Abán CE, Accialini PL, Etcheverry T, Leguizamón GF, Martinez NA (2018) Farina MG crosstalk between nitric oxide and endocannabinoid signaling pathways in normal and pathological placentation. Front Physiol 9:1699
Knöfler M, Haider S, Saleh L, Pollheimer J, Gamage TKJB, James J (2019) Human placenta and trophoblast development: key molecular mechanisms and model systems. Cell Mol Life Sci 76:3479–3496
Afkham A, Eghbal-Fard S, Heydarlou H, Azizi R, Aghebati-Maleki L, Yousefi M (2019) Toll-like receptors signaling network in pre-eclampsia: An updated review. J Cell Physiol 234:2229–2240
Zhang Z, Wang X, Zhang L, Shi Y, Wang J, Yan H (2017) Wnt/β-catenin signaling pathway in trophoblast cells and abnormal activation in preeclampsia (Review). Mol Med Rep 16:1007–1013
Gupta SK, Malhotra SS, Malik A, Verma S, Chaudhary P (2016) Cell signaling pathways involved during invasion and syncytialization of trophoblast cells. Am J Reprod Immunol 75:361–371
Mo L, Hong S, Li Y, Hu Z, Han B, Wei Z, Jia J (2020) Sevoflurane inhibited inflammatory response induced by TNF-α in human trophoblastic cells through p38MAPK signaling pathway. J Recept Signal Transduct Res 18:1–6
Dard L, Bellance N, Lacombe D, Rossignol R (2018) RAS signalling in energy metabolism and rare human diseases. Biochim Biophys Acta Bioenerg 1859:845–867
Al-Olabi L, Polubothu S, Dowsett K, Andrews KA, Stadnik P, Joseph AP, Knox R, Pittman A, Clark G, Baird W et al (2018) Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest 128:5185
Zeng X, Hunt A, Jin SC, Duran D, Gaillard J, Kahle KT (2019) EphrinB2-EphB4-RASA1 signaling in human cerebrovascular development and disease. Trends Mol Med 25:265–286
Sung H, Kanchi KL, Wang X, Hill KS, Messina JL, Lee JH, Kim Y, Dees ND, Ding L, Teer JK et al (2016) Inactivation of RASA1 promotes melanoma tumorigenesis via R-Ras activation. Oncotarget 7:23885–23896
Li L, Fan Y, Huang X, Luo J, Zhong L, Shu XS, Lu L, Xiang T, Chan ATC, Yeo W et al (2019) Tumor suppression of ras GTPase-activating protein RASA5 through antagonizing ras signaling perturbation in carcinomas. Science 21:1–18
Liu M, Wang Y, Lu H, Wang H, Shi X, Shao X, Li YX, Zhao Y, Wang YL (2018) miR-518b enhances human trophoblast cell proliferation through targeting Rap1b and activating Ras-MAPK signal. Front Endocrinol (Lausanne) 9:100
Arthurs AL, Lumbers ER, Pringle KG (2019) MicroRNA mimics that target the placental renin-angiotensin system inhibit trophoblast proliferation. Mol Hum Reprod 25:218–227
Liu C, Liang X, Wang J, Zheng Q, Zhao Y, Khan MN, Liu S, Yan Q (2017) Protein O-fucosyltransferase 1 promotes trophoblast cell proliferation through activation of MAPK and PI3K/Akt signaling pathways. Biomed Pharmacother 88:95–101
Zhu HY, Wang JX, Tong XM, Xue YM, Zhang SY (2015) S100P regulates trophoblast-like cell proliferation via P38 MAPK pathway. Gynecol Endocrinol 31:796–800
Wang Z, Liu M, Nie X, Zhang Y, Chen Y, Zhu L, Chen X, Chen L, Chen H, Zhang J (2015) NOD1 and NOD2 control the invasiveness of trophoblast cells via the MAPK/p38 signaling pathway in human first-trimester pregnancy. Placenta 36:652–660
Post JB, Roodhart JML, Snippert HJG (2020) Colorectal cancer modeling with organoids: discriminating between oncogenic RAS and BRAF variants. Trends Cancer 6:111–129
Harrell Stewart DR, Clark GJ (2020) Pumping the brakes on RAS - negative regulators and death effectors of RAS. J Cell Sci. https://doi.org/10.1242/jcs.238865
Deng S, Clowers MJ, Velasco WV, Ramos-Castaneda M, Moghaddam SJ (2020) Understanding the complexity of the tumor microenvironment in K-ras mutant lung cancer: finding an alternative path to prevention and treatment. Front Oncol 9:1556
Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK (1993) Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 206:204–211
Abou-Kheir W, Barrak J, Hadadeh O, Daoud G (2017) HTR-8/SVneo cell line contains a mixed population of cells. Placenta 50:1–7
Chen D, Teng JM, North PE, Lapinski PE, King PD (2019) RASA1-dependent cellular export of collagen IV controls blood and lymphatic vascular development. J Clin Invest 130:3545–3561
Degirmenci U, Wang M, Hu J (2020) Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells 9:198
Funding
The Project Supported by The Natural Science Foundation of Guangdong Province (Grant No. 2018A0303100021), National Natural Science Foundation of China (Grant No. 81971385), National Natural Science Foundation of China (Grant No. 81902751), Natural Science Foundation of Guangdong Province (Grant No. 2019A1515010412) and Researcher Cultivation Project of Shenzhen People's Hospital (Grant No. SYKYPY201927).
Author information
Authors and Affiliations
Contributions
ZJ and GYL researched conception and design. ZJ analyzed data and interpretation. GYL and LXQ analyzed statistically. ZJ and GYL drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
No potential conflicts of interest were disclosed.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethic committee of the Shenzhen People’s Hospital.
Consent to participate
All patients in the present study provided written informed consent.
Consent for publication
All authors have read the manuscript and approved the final version.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
About this article
Cite this article
Zhang, J., Liu, X. & Gao, Y. Abnormal H3K27 histone methylation of RASA1 gene leads to unexplained recurrent spontaneous abortion by regulating Ras-MAPK pathway in trophoblast cells. Mol Biol Rep 48, 5109–5119 (2021). https://doi.org/10.1007/s11033-021-06507-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06507-6