Abstract
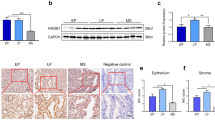
Recurrent implantation failure (RIF) is diagnosed when good-quality embryos repeatedly fail to implant after transfer in several in vitro fertilization (IVF) treatment cycles. Different expression profiles in maternal mRNAs could be referring to many diseases including RIF. This study aimed to reveal significantly dysregulated selected genes expression between healthy fertile women and RIF patients in the implantation window days of the natural menstrual cycle. MME, WWC1, TNC, and FOXP3 genes were chosen as target genes regarding their possible relations with the implantation process. Pathways with these genes were identified and the relationship between these pathways and RIF was investigated. In this study, the endometrial biopsy samples were collected in the secretory phase (cycle day 20–24) of the menstrual cycle from RIF patients (n = 34) and healthy fertile controls (n = 34). After “Pathway and network-oriented GWAS analysis” (PANOGA) and “Kyoto Encyclopedia of Genes and Genomes” (KEGG) pathway analysis; “Membrane Metalloendopeptidase” (MME), “WW and C2 Domain Containing 1” (WWC1), “Tenascin C” (TNC) and “Forkhead Box P3” (FOXP3) genes were chosen as target genes by regarding their possible relation with implantation process. Detection of differences in mRNA expressions between the control group and RIF patients has been performed with the droplet digital PCR (ddPCR) method. Results of the study showed that MME and WWC1 genes expression levels are significantly (p < 0,05) up-regulated 4.9 and 5.2 times respectively and TNC gene expression level is significantly (p < 0,05) down-regulated 9 times in the RIF samples compared to the control group. However, no statistically significant difference was observed between the patient group and the control group in the expression of the FOXP3 gene (p < 0.05). Changes are observed in the expression of the renin-angiotensin system pathway in which the MME gene is involved in the implantation process. The increase in MME gene expression can be speculated to cause implantation failure by restricting the invasion of trophoblast cells. Increasing WWC1 gene expression in the Hippo signaling pathway inhibits “Yes-associated protein 1” (YAP) expression, which is a transcriptional cofactor. Inhibition of YAP protein expression may impair the implantation process by causing the failure of endometrial decidualization. The TNC gene is located in the focal adhesion pathway and this pathway reduces cell adhesion on the endometrial surface to facilitate the attachment of the embryo to the endometrium. The reason for implantation failure might be that the intercellular connections are not suitable for implantation as a result of decreased expression of the focal adhesion pathway in which the TNC gene is effective. Considering the relations between the pathways of the target genes and the implantation process, changes in the expression of target genes might be a cause of RIF.



Similar content being viewed by others
Data availability
All analyzed and generated data in the study are included in this paper. The clinical datasets are not publicly available regarding ethical responsibility to respect participants’ privacy rights.
References
Simon A, Laufer N (2012) Repeated implantation failure: Clinical approach. Fertil Steril 97:1039–1043. https://doi.org/10.1016/j.fertnstert.2012.03.010
Simón C, Martín JC, Pellicer A (2000) Paracrine regulators of implantation. Bailliere’s Best Pract Res Clin Obstet Gynaecol 14:815–826. https://doi.org/10.1053/beog.2000.0121
Coutifaris C, Myers ER, Guzick DS et al (2004) Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 82:1264–1272. https://doi.org/10.1016/j.fertnstert.2004.03.069
Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T (2006) Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod 21:3036–3043. https://doi.org/10.1093/humrep/del305
Stern JE, Cedars MI, Jain T et al (2007) Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril 88:275–282. https://doi.org/10.1016/j.fertnstert.2006.09.016
Chian RC, Nargund G, Huang JYJ (2017) Development of in vitro maturation for human oocytes: natural and mild approaches to clinical infertility treatment. Springer, Cham
Maruyama T, Yoshimura Y (2008) Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 55:795–810
Krüssel JS, Bielfeld P, Polan ML, Simón C (2003) Regulation of embryonic implantation. Eur J Obstet Gynecol Reprod Biol 110:S2. https://doi.org/10.1016/S0301-2115(03)00167-2
Goodman C, Jeyendran RS, Coulam CB (2008) Vascular endothelial growth factor gene polymorphism and implantation failure. Reprod Biomed Online 16:720–723. https://doi.org/10.1016/S1472-6483(10)60487-7
Mojarrad M, Hassanzadeh-Nazarabadi M, Tafazoli N (2013) Polymorphism of genes and implantation failure. Int J Mole Cell Med 2:1–8
Bastu E, Demiral I, Gunel T et al (2019) Potential marker pathways in the endometrium that may cause recurrent implantation failure. Reprod Sci 26:879–890. https://doi.org/10.1177/1933719118792104
Kresowik JDK, Devor EJ, Van Voorhis BJ, Leslie KK (2014) MicroRNA-31 Is significantly elevated in both human endometrium and serum during the window of implantation: a potential biomarker for optimum receptivity. Biol Reprod. https://doi.org/10.1095/biolreprod.113.116590
Jahan P, Sreenivasagari R, Goudi D et al (2013) Role of Foxp3 gene in maternal susceptibility to pre-eclampsia—a study from South India. Scand J Immunol 77:104–108. https://doi.org/10.1111/j.1365-3083.2012.02760.x
Azhari F, Pence S, Hosseini MK et al (2020) The role of the serum exosomal and endometrial microRNAs in recurrent implantation failure. J Mater Fetal Neonatal Med. https://doi.org/10.1080/14767058.2020.1849095
Kanehisa M, Furumichi M, Tanabe M et al (2017) KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45:D353–D361. https://doi.org/10.1093/nar/gkw1092
Fatemi HM, Popovic-Todorovic B (2013) Implantation in assisted reproduction: a look at endometrial receptivity. Reprod Biomed Online 27:530–538. https://doi.org/10.1016/j.rbmo.2013.05.018
Potdar N, Gelbaya T, Nardo LG (2012) Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta-analysis. Reprod Biomed Online 25:561–571. https://doi.org/10.1016/j.rbmo.2012.08.005
Ledee-Bataille N (2002) Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod 17:213–218. https://doi.org/10.1093/humrep/17.1.213
GeneCards MME Gene & Pathways. https://www.genecards.org/cgi-bin/carddisp.pl?gene=MME#pathways_interactions. Accessed 17 Tem 2020
Anton L, Brosnihan KB (2008) Systemic and uteroplacental renin—angiotensin system in normal and pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis 2:349–362. https://doi.org/10.1177/1753944708094529
Lourenço ELB, Muller JC, Boareto AC et al (2014) Effects of angiotensin-converting enzyme inhibitor derived from Tropaeolum majus L. in Rat preimplantation embryos: evidence for the dehydroepiandrosterone and estradiol role. Evid Based Complement Altern Med. https://doi.org/10.1155/2014/209207
Xia Y, Wen HY, Kellems RE (2002) Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J Biol Chem 277:24601–24608. https://doi.org/10.1074/jbc.M201369200
GeneCards WWC1 Gene & Pathways. https://www.genecards.org/cgi-bin/carddisp.pl?gene=WWC1&keywords=WWC1#pathways_interactions. Accessed 17 Tem 2020
Zhu HY, Ge TX, Bin PY, Zhang SY (2017) Advanced role of hippo signaling in endometrial fibrosis: implications for intrauterine adhesion. Chin Med J 130:2732–2737. https://doi.org/10.4103/0366-6999.218013
Strakova Z, Kruss S, Morris K, Reed J (2010) Members of the hippo pathway are regulated in the uterus during the menstrual cycle. Biol Reprod 83:363–363. https://doi.org/10.1093/biolreprod/83.s1.363
Chen H, Song Y, Yang S et al (2017) YAP Mediates human decidualization of the uterine endometrial stromal cells. Placenta 53:30–35. https://doi.org/10.1016/j.placenta.2017.03.013
GeneCards TNC Gene & Pathways. https://www.genecards.org/cgi-bin/carddisp.pl?gene=TNC&keywords=TNC#pathways_interactions. Accessed 17 Tem 2020
Burridge K, Fath K, Kelly T et al (1988) Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol 4:487–525. https://doi.org/10.1146/annurev.cb.04.110188.002415
Lo SH (2006) Focal adhesions: What’s new inside. Dev Biol 294:280–291. https://doi.org/10.1016/j.ydbio.2006.03.029
Chen Q, Zhang A, Yu F et al (2015) Label-free proteomics uncovers energy metabolism and focal adhesion regulations responsive for endometrium receptivity. J Proteome Res 14:1831–1842. https://doi.org/10.1021/acs.jproteome.5b00038
Kaneko Y, Lecce L, Murphy CR (2009) Ovarian hormones regulate expression of the focal adhesion proteins, talin and paxillin, in rat uterine luminal but not glandular epithelial cells. Histochem Cell Biol 132:613–622. https://doi.org/10.1007/s00418-009-0641-x
Nicholson MDO, Lindsay LA, Murphy CR (2010) Ovarian hormones control the changing expression of claudins and occludin in rat uterine epithelial cells during early pregnancy. Acta Histochem 112:42–52. https://doi.org/10.1016/j.acthis.2008.07.003
Jalali BM, Likszo P, Andronowska A, Skarzynski DJ (2018) Alterations in the distribution of actin and its binding proteins in the porcine endometrium during early pregnancy: possible role in epithelial remodeling and embryo adhesion. Theriogenology 116:17–27. https://doi.org/10.1016/j.theriogenology.2018.05.004
Erikson DW, Burghardt RC, Bayless KJ, Johnson GA (2009) Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alphavbeta6 on porcine trophectoderm cells and integrin alphavbeta3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration1. Biol Reprod 81:814–825. https://doi.org/10.1095/biolreprod.109.078600
Michie HJ, Head JR (1994) Tenascin in pregnant and non-pregnant rat uterus: Unique spatio-temporal expression during decidualization. Biol Reprod 50:1277–1286. https://doi.org/10.1095/biolreprod50.6.1277
Kida H (1997) The change in tenascin expression in mouse uterus during early pregnancy. J Assist Reprod Genet 14:44–50. https://doi.org/10.1007/BF02765752
Noda N, Minoura H, Nishiura R et al (2000) Expression of Tenascin-C in stromal cells of the murine uterus during early pregnancy: Induction by interleukin-1α, prostaglandin E2, and prostaglandin F(2α). Biol Reprod 63:1713–1720. https://doi.org/10.1095/biolreprod63.6.1713
Koler M, Achache H, Tsafrir A et al (2009) Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod 24:2541–2548. https://doi.org/10.1093/humrep/dep193
Funding
This study was supported by the Istanbul University Scientific Research Projects Department (Grant No. 32431).
Author information
Authors and Affiliations
Contributions
BKB and EB contributed to patient selection and collecting samples. EÜ and US contributed to the target gene selection part. TG designed the molecular genetics experiments. İGA, FA, and ENÇ performed the RNA isolation and ddPCR. İGA performed data analysis. Each author participated in manuscript editing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the Ethical Committee of Istanbul Medical Faculty (Istanbul, Turkey; Project No: 2018/1373).
Consent to participate
All the study participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albayrak, İ.G., Azhari, F., Çolak, E.N. et al. Endometrial gene expression profiling of recurrent implantation failure after in vitro fertilization. Mol Biol Rep 48, 5075–5082 (2021). https://doi.org/10.1007/s11033-021-06502-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06502-x