Abstract
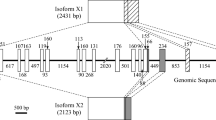
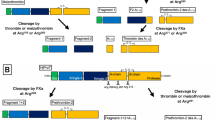
Kalliklectin is a unique fish-specific lectin that demonstrates sequence similarity to mammalian plasma kallikrein and coagulation factor XI, which are not lectins but proteases. Reported fish kalliklectins and these mammalian proteases comprise four characteristic “apple domains” (APDs). Bioinformatics analysis revealed that Siluriformes species possess anomalous kalliklectins comprising 6 to 16 APDs. Complementary DNA cloning showed that the full-length nucleotide sequence of Ictalurus punctatus consists of 2240 bp that encode 720 amino acid residues to produce a mature protein with a putative 18 amino acid N-terminus peptide sequence. This protein has a predicted molecular mass of 83,417.23 Da. Reverse transcription-polymerase chain reaction (RT-PCR) showed that this lectin gene expresses in the liver but not in any other tissues, including the mucosal tissues. This differential expression pattern makes this lectin unique compared to other lectins described in previous studies. We successfully detected an 85-kDa protein in the serum using western blotting analysis, suggesting that this lectin protein is produced by the liver and secreted into the bloodstream. We characterized a novel cDNA sequence encoding a new type of kalliklectin with eight APDs isolated from channel catfish, I. punctatus. Based on phylogenetic analysis, we speculated that there was a duplication of the third and fourth APD set in a common Siluriformes ancestor at some point after its separation from the common teleost ancestor and that these duplications then underwent independent repeats in different lineages resulting in the generation of the [APD1]-[APD2]-{[APD3]-[APD-4]} × n structure in modern catfishes.




Similar content being viewed by others
References
Sharon N, Lis H (1972) Lectins: cell-agglutinating and sugar-specific proteins. Science 177(4053):949–959. https://doi.org/10.1126/science.177.4053.949
Kubo T, Kawasaki K, Natori S (1990) Sucrose-binding lectin in regenerating cockroach (Periplaneta americana) legs: its purification from adult hemolymph. Insect Biochem 20(6):585–591. https://doi.org/10.1016/0020-1790(90)90070-B
Ni Y, Tizard I (1996) Lectin carbohydrate interaction in the immune system. Vet Immunol Immunopathol 55(1–3):205–223. https://doi.org/10.1016/s0165-2427(96)05718-2
Jimbo M, Yanohara T, Koike K, Koike K, Sakai R, Muramoto K, Kamiya H (2000) The d-galactose-binding lectin of the octocoral Sinularia lochmodes: characterization and possible relationship to the symbiotic dinoflagellates. Comp Biochem Physiol B 125(2):227–236. https://doi.org/10.1016/s0305-0491(99)00173-x
Springer SA, Moy GW, Friend DS, Swanson WJ, Vacquier VD (2008) Oyster sperm bindin is a combinatorial fucose lectin with remarkable intra-species diversity. Int J Dev Biol 52(5–6):759–768. https://doi.org/10.1387/ijdb.082581ss
Dan H, Tateno H, Sato T, Narimatsu H, Hirabayashi J (2013) Tailoring GalNAcα1–3Galβ-specific lectins from a multi-specific fungal galectin: dramatic change of carbohydrate specificity by a single amino-acid substitution. Biochem J 453(2):261–270. https://doi.org/10.1042/BJ20121901
Vasta GR, Ahmed H (2008) Animal lectins: a functional view. CRC Press, Boca Raton
Tsutsui S, Okamoto M, Ono M, Suetake H, Kikuchi K, Nakamura O, Suzuki Y, Watanabe T (2011) A new type of lectin discovered in a fish, flathead (Platycephalus indicus), suggests an alternative functional role for mammalian plasma kallikrein. Glycobiology 21:1580–1587. https://doi.org/10.1093/glycob/cwr070
Tsutsui S, Yamamura N, Yoshida T, Nakamura O (2015) Fugu (Takifugu rubripes) serum GlcNAc-binding lectin is a kalliklectin but has different properties from those of a reported homologue. J Biochem 158:189–195. https://doi.org/10.1093/jb/mvv033
Wuepper KD, Cochrane CG (1972) Plasma prekallikrein: Isolation, characterization, and mechanism of activation. J Exp Med 135:1–20. https://doi.org/10.1084/jem.135.1.1
Fujikawa K, Chung DW, Hendrickson LE, Davie EW (1986) Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochem 25(9):2417–2424. https://doi.org/10.1021/bi00357a018
Chung DW, Fujikawa K, McMullen BA, Davie EW (1986) Human plasma prekallikrein, a zymogen to a serine protease that contains four tandem repeats. Biochem 25(9):2410–2417. https://doi.org/10.1021/bi00357a017
Werle E, Götze W, Keppler A (1937) Über die wirkung des kallikreins auf den isolierten darm und über eine neue darmkontrahierende substanz. Biochem Z 289:217–233
Golias Ch, Charalabopoulos A, Stagikas D, Charalabopoulos K, Batistatou A (2007) The kinin system-bradykinin: biological effects and clinical implications. Multiple role of the kinin system-bradykinin. Hippokratia 11(3):124–128
Petho G, Reeh PW (2012) Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev 92(4):1699–1775. https://doi.org/10.1152/physrev.00048.2010
Wong MK, Takei Y (2013) Lack of plasma kallikrein-kinin system cascade in teleosts. PLoS ONE 8:e81057. https://doi.org/10.1371/journal.pone.0081057
Jiang Y, Doolittle RF (2003) The evolution of vertebrate blood coagulation as viewed from a comparison of puffer fish and sea squirt genomes. Proc Natl Acad Sci USA 100(13):7527–7532. https://doi.org/10.1073/pnas.0932632100
Ponczec MB, Gailani D, Doolittle RF (2008) Evolution of the contact phase of vertebrate blood coagulation. J Thromb Haemostasis 6:1–8. https://doi.org/10.1111/j.1538-7836.2008.03143.x
Doolittle RF (2011) (2011) Coagulation in vertebrates with a focus on evolution and inflammation. J Innate Immun 3:9–16. https://doi.org/10.1159/000321005
Tsutsui S, Suzuki Y, Shibuya K, Nakamura O (2018) Sacciform cells in the epidermis of fugu (Takifugu rubripes) produce and secrete kalliklectin, a novel lectin found in teleosts. Fish Shellfish Immunol 80:311–318. https://doi.org/10.1016/j.fsi.2018.06.017
Volff JN (2005) Genome evolution and biodiversity in teleost fish. Heredity 94(3):280–294. https://doi.org/10.1038/sj.hdy.6800635
Small BC, Quiniou SMA (2018) Characterization of two channel catfish, Ictalurus punctatus, glucocorticoid receptors and expression following an acute stressor. Comp Biochem Physiol A Mol Integr Physiol 216:42–51. https://doi.org/10.1016/j.cbpa.2017.11.011
Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, Sasamoto S, Watanabe A, Idesawa K, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kiyokawa C, Kohara M, Matsumoto M, Matsuno A, Mochizuki Y, Nakayama S, Nakazaki N, Shimpo S, Sugimoto M, Takeuchi C, Yamada M, Tabata S (2000) Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res 7:331–338. https://doi.org/10.1093/dnares/7.6.331
Brown PJ, Billington KJ, Bumstead JM, Clark JD, Tomley FM (2000) A microneme protein from Eimeria tenella with homology to the apple domains of coagulation factor XI and plasma pre-kallikrein. Mol Biochem Parasitol 107:91–102. https://doi.org/10.1016/s0166-6851(00)00179-1
Brecht S, Carruthers VB, Ferguson DJ, Giddings OK, Wang G, Jakle U, Harper JM, Sibley LD, Soldati D (2001) The Toxoplasma micronemal protein MIC4 is an adhesin composed of six conserved apple domains. J Biol Chem 276:4119–4127. https://doi.org/10.1074/jbc.M008294200
Mateos F, Rickauer M, Esquerre-Tugaye MT (1997) Cloning and characterization of a cDNA encoding an elicitor of Phytophthora parasitica var. nicotianae that shows cellulose-binding and lectin-like activities. Mol Plant Microbe Interact 10:1045–1053. https://doi.org/10.1094/MPMI.1997.10.9.1045
Walker JC, Zhang R (1990) Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature 345:743–746. https://doi.org/10.1038/345743a0
Tordai H, Ba´nyai L, Patthy L (1999) The PAN module: the N-terminal domains of plasminogen and hepatocyte growth factor are homologous with the apple domains of the prekallikrein family and with a novel domain found in numerous nematode proteins. FEBS Lett 461:63–67. https://doi.org/10.1016/s0014-5793(99)01416-7
Matsui S, Yoshikawa S, Suzuki S, Somamoto T, Yamamoto A, Naoakura O, Tsutsui S (2020) Expression profile of kalliklectin, a soluble-type mannose receptor, during embryogenesis and early larval development in fugu (Takifugu rubripes). Mol Immunol 126:129–135. https://doi.org/10.1016/j.molimm.2020.07.022
Acknowledgements
We thank Namazuya Inc., a fish farm in Lake Kasumigaura, Ibaraki, Japan, for providing animals.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
ST designed the study. ST performed bioinfomatic analysis and cDNA cloning. AY took care of PCR and western blot analyses. YK discovered the new type for the first time. ST wrote the paper with input from all authors. ON discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest directly relevant to the content of this article.
Ethical approval
This study was approved by the Kitasato University Safety Committee for Recombinant DNA Experiments (Permission Number: 4536).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsutsui, S., Yoshimura, A., Kawakami, Y. et al. Molecular evolution of kalliklectin in teleost and identification of the novel type with eight apple domains in channel catfish, Ictalurus punctatus. Mol Biol Rep 48, 4305–4318 (2021). https://doi.org/10.1007/s11033-021-06446-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06446-2