Abstract
Neuronal senescence, triggered by telomere shortening, oncogene activation, DNA damage, or oxidative stress, has been associated with neurodegenerative diseases’ pathogenesis. Therefore, preventing neuronal senescence could be a novel treatment strategy for neurodegenerative diseases. Lithium (Li), the first-line treatment against bipolar disorder, has been shown to have neuroprotective effects in clinical, pre-clinical, and in vitro studies. Li can protect cells from senescence, and its effect on neuronal senescence was investigated in our study. Furthermore, we also investigated the effects of Li on the senescence-associated miR-34a/Sirt1/p53 pathway. In this study, hydrogen peroxide was used as an inducer for the “stress-induced premature senescence” model. In the senescence model, we have assessed Li’s effects on senescence by analyzing β-galactosidase activity, Sudan Black B, and senescence-associated heterochromatin foci (SAHF) stainings, and on cell cycle arrest by BrdU staining. Furthermore, expression levels of senescence and cell cycle arrest-related proteins (p53, p21, p16INK4a, and SIRT1) by western blotting. Finally, the effects of Li on senescence-associated miR-34a levels were measured by quantitative PCR. We show via Sudan Black B staining, β-Gal activity assay, and by detecting SAHF, Li protects against senescence in neuronal cells. Then, Li’s effect on signaling has also been determined on pathways involved in senescence and cell cycle arrest. Moreover, we have observed that Li has a modulatory effect on miR-34a expression. Therefore, we posit that Li suppresses senescence in neuronal cells and that this effect is mediated through miR-34a/Sirt1/p53 axis.

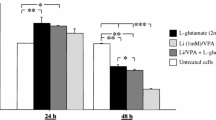
taken from a BrdU incorporation assay following Li and H2O2 treatment. Data are mean ± SEM, n = 5 (**p < 0.01 vs. control; #p < 0.05 vs. H2O2). (Color figure online)




Similar content being viewed by others
References
Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21(12):1424–1435. https://doi.org/10.1038/nm.4000
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24(22):2463–2479. https://doi.org/10.1101/gad.1971610
Chen JH, Hales CN, Ozanne SE (2007) DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res 35(22):7417–7428. https://doi.org/10.1093/nar/gkm681
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120(4):513–522. https://doi.org/10.1016/j.cell.2005.02.003
van Deursen JM (2014) The role of senescent cells in ageing. Nature 509(7501):439–446. https://doi.org/10.1038/nature13193
Campisi J, d’Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740. https://doi.org/10.1038/nrm2233
Wang AS, Dreesen O (2018) Biomarkers of cellular senescence and skin aging. Front Genet 9:247. https://doi.org/10.3389/fgene.2018.00247
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92(20):9363–9367. https://doi.org/10.1073/pnas.92.20.9363
Piechota M, Sunderland P, Wysocka A, Nalberczak M, Sliwinska MA, Radwanska K, Sikora E (2016) Is senescence-associated beta-galactosidase a marker of neuronal senescence? Oncotarget 7(49):81099–81109. https://doi.org/10.18632/oncotarget.12752
Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Saffrey MJ, Cameron K, von Zglinicki T (2012) Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11(6):996–1004. https://doi.org/10.1111/j.1474-9726.2012.00870.x
Martinez-Cue C, Rueda N (2020) Cellular senescence in neurodegenerative diseases. Front Cell Neurosci 14:16. https://doi.org/10.3389/fncel.2020.00016
Kritsilis M, Rizou SV, Koutsoudaki P, Evangelou K, Gorgoulis V, Papadopoulos D (2018) Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci 19(10):2937. https://doi.org/10.3390/ijms19102937
Yang Y, Zheng A, Li M, Duan W, Mu X, Wang X (2016) Medical economic burden of the ageing population: a multistage sampling analysis of 3 532 517 cases. Lancet 388:S79. https://doi.org/10.1016/s0140-6736(16)32006-2
Diniz BS, Machado-Vieira R, Forlenza OV (2013) Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat 9:493–500. https://doi.org/10.2147/NDT.S33086
Alural B, Ozerdem A, Allmer J, Genc K, Genc S (2015) Lithium protects against paraquat neurotoxicity by NRF2 activation and miR-34a inhibition in SH-SY5Y cells. Front Cell Neurosci 9:209. https://doi.org/10.3389/fncel.2015.00209
Taler M, Aronovich R, Henry Hornfeld S, Dar S, Sasson E, Weizman A, Hochman E (2021) Regulatory effect of lithium on hippocampal blood-brain barrier integrity in a rat model of depressive-like behavior. Bipolar Disord 23(1):55–65. https://doi.org/10.1111/bdi.12962
Li M, Xia M, Chen W, Wang J, Yin Y, Guo C, Li C, Tang X, Zhao H, Tan Q, Chen Y, Jia Z, Liu X, Feng H (2020) Lithium treatment mitigates white matter injury after intracerebral hemorrhage through brain-derived neurotrophic factor signaling in mice. Transl Res 217:61–74. https://doi.org/10.1016/j.trsl.2019.12.006
Kirkland JL, Tchkonia T (2017) Cellular senescence: a translational perspective. EBioMedicine 21:21–28. https://doi.org/10.1016/j.ebiom.2017.04.013
Ryves WJ, Harwood AJ (2001) Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun 280(3):720–725. https://doi.org/10.1006/bbrc.2000.4169
Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS (2003) Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem 278(49):48872–48879. https://doi.org/10.1074/jbc.M305870200
Zmijewski JW, Jope RS (2004) Nuclear accumulation of glycogen synthase kinase-3 during replicative senescence of human fibroblasts. Aging Cell 3(5):309–317. https://doi.org/10.1111/j.1474-9728.2004.00117.x
Wei YB, Backlund L, Wegener G, Mathe AA, Lavebratt C (2015) Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium. Int J Neuropsychopharmacol 18(7):pyv002. https://doi.org/10.1093/ijnp/pyv002
Struewing IT, Durham SN, Barnett CD, Mao CD (2009) Enhanced endothelial cell senescence by lithium-induced matrix metalloproteinase-1 expression. J Biol Chem 284(26):17595–17606. https://doi.org/10.1074/jbc.M109.001735
Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J (2010) Enhancement of intervertebral disc cell senescence by WNT/beta-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum 62(10):3036–3047. https://doi.org/10.1002/art.27599
Viel T, Chinta S, Rane A, Chamoli M, Buck H, Andersen J (2020) Microdose lithium reduces cellular senescence in human astrocytes—a potential pharmacotherapy for COVID-19? Aging 12(11):10035–10040. https://doi.org/10.18632/aging.103449
Suh N (2018) MicroRNA controls of cellular senescence. BMB Rep 51(10):493–499
Maes OC, Sarojini H, Wang E (2009) Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J Cell Physiol 221(1):109–119. https://doi.org/10.1002/jcp.21834
Xu X, Chen W, Miao R, Zhou Y, Wang Z, Zhang L, Wan Y, Dong Y, Qu K, Liu C (2015) miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget 6(6):3988–4004. https://doi.org/10.18632/oncotarget.2905
Yamakuchi M (2012) MicroRNA regulation of SIRT1. Front Physiol 3:68. https://doi.org/10.3389/fphys.2012.00068
Tsurumi A, Li WX (2012) Global heterochromatin loss: a unifying theory of aging? Epigenetics 7(7):680–688. https://doi.org/10.4161/epi.20540
Fulzele S, Mendhe B, Khayrullin A, Johnson M, Kaiser H, Liu Y, Isales CM, Hamrick MW (2019) Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging 11(6):1791–1803. https://doi.org/10.18632/aging.101874
Li X, Khanna A, Li N, Wang E (2011) Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging. Aging 3(10):985–1002. https://doi.org/10.18632/aging.100371
Owczarz M, Budzinska M, Domaszewska-Szostek A, Borkowska J, Polosak J, Gewartowska M, Slusarczyk P, Puzianowska-Kuznicka M (2017) miR-34a and miR-9 are overexpressed and SIRT genes are downregulated in peripheral blood mononuclear cells of aging humans. Exp Biol Med 242(14):1453–1461. https://doi.org/10.1177/1535370217720884
Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J (2013) The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154(2):430–441. https://doi.org/10.1016/j.cell.2013.06.016
Baviskar SN (2011) A quick andautomated method for measuring cell area using imageJ. Am Biol Teach 73(9):554–556. https://doi.org/10.1525/abt.2011.73.9.9
Hirano T, Murakami T, Ono H, Sakurai A, Tominaga T, Takahashi T, Nagai K, Doi T, Abe H (2015) A novel interaction between FLICE-associated huge protein (FLASH) and E2A regulates cell proliferation and cellular senescence via tumor necrosis factor (TNF)-alpha-p21WAF1/CIP1 axis. PLoS ONE 10(7):e0133205. https://doi.org/10.1371/journal.pone.0133205
Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J, Serrano M, Gorgoulis VG (2013) Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging 5(1):37–50. https://doi.org/10.18632/aging.100527
Evangelou K, Gorgoulis VG (2017) Sudan Black B, the specific histochemical stain for lipofuscin: a novel method to detect senescent cells. Methods Mol Biol 1534:111–119. https://doi.org/10.1007/978-1-4939-6670-7_10
Santacruz-Perez C, Tonolli PN, Ravagnani FG, Baptista MS (2018) Photochemistry of lipofuscin and the interplay of UVA and Visible light in skin photosensitivity. In: Saha S, Mondal S (eds) Photochemistry and photophysics—fundamentals to applications. IntechOpen, London
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Aird KM, Zhang R (2013) Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol Biol 965:185–196. https://doi.org/10.1007/978-1-62703-239-1_12
Wang Z, Wei D, Xiao H (2013) Methods of cellular senescence induction using oxidative stress. Methods Mol Biol 1048:135–144. https://doi.org/10.1007/978-1-62703-556-9_11
Han SM, Kim JM, Park KK, Chang YC, Pak SC (2014) Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Complement Altern Med 14:286. https://doi.org/10.1186/1472-6882-14-286
Chetsawang J, Govitrapong P, Chetsawang B (2010) Hydrogen peroxide toxicity induces Ras signaling in human neuroblastoma SH-SY5Y cultured cells. J Biomed Biotechnol. https://doi.org/10.1155/2010/803815
Panossian L, Fenik P, Zhu Y, Zhan G, McBurney MW, Veasey S (2011) SIRT1 regulation of wakefulness and senescence-like phenotype in wake neurons. J Neurosci 31(11):4025–4036. https://doi.org/10.1523/JNEUROSCI.5166-10.2011
Moreno-Blas D, Gorostieta-Salas E, Pommer-Alba A, Mucino-Hernandez G, Geronimo-Olvera C, Maciel-Baron LA, Konigsberg M, Massieu L, Castro-Obregon S (2019) Cortical neurons develop a senescence-like phenotype promoted by dysfunctional autophagy. Aging (Albany NY) 11(16):6175–6198. https://doi.org/10.18632/aging.102181
Duan J, Duan J, Zhang Z, Tong T (2005) Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol 37(7):1407–1420. https://doi.org/10.1016/j.biocel.2005.01.010
Wan C, Liu J, Nie X, Zhao J, Zhou S, Duan Z, Tang C, Liang L, Xu G (2014) 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS ONE 9(2):e89811. https://doi.org/10.1371/journal.pone.0089811
Kumari R, Jat P (2021) Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol 9:645593. https://doi.org/10.3389/fcell.2021.645593
Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A (2020) Role of p53 in the regulation of cellular senescence. Biomolecules 10(3):420. https://doi.org/10.3390/biom10030420
Zhou N, Lin X, Dong W, Huang W, Jiang W, Lin L, Qiu Q, Zhang X, Shen J, Song Z, Liang X, Hao J, Wang D, Hu Z (2016) SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci Rep 6:22628. https://doi.org/10.1038/srep22628
Huang J, Gan Q, Han L, Li J, Zhang H, Sun Y, Zhang Z, Tong T (2008) SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS ONE 3(3):e1710. https://doi.org/10.1371/journal.pone.0001710
Okada N, Lin CP, Ribeiro MC, Biton A, Lai G, He X, Bu P, Vogel H, Jablons DM, Keller AC, Wilkinson JE, He B, Speed TP, He L (2014) A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes Dev 28(5):438–450. https://doi.org/10.1101/gad.233585.113
Kulikov R, Boehme KA, Blattner C (2005) Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Mol Cell Biol 25(16):7170–7180. https://doi.org/10.1128/MCB.25.16.7170-7180.2005
Dwivedi T, Zhang H (2014) Lithium-induced neuroprotection is associated with epigenetic modification of specific BDNF gene promoter and altered expression of apoptotic-regulatory proteins. Front Neurosci 8:457. https://doi.org/10.3389/fnins.2014.00457
Wang J, Feng H, Zhang J, Jiang H (2013) Lithium and valproate acid protect NSC34 cells from H2O2-induced oxidative stress and upregulate expressions of SIRT3 and CARM1. Neuro Endocrinol Lett 34(7):648–654
Hoshi M, Sato M, Matsumoto S, Noguchi A, Yasutake K, Yoshida N, Sato K (2003) Spherical aggregates of beta-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3beta. Proc Natl Acad Sci USA 100(11):6370–6375. https://doi.org/10.1073/pnas.1237107100
Kang C (2019) Senolytics and senostatics: a two-pronged approach to target cellular senescence for delaying aging and age-related diseases. Mol Cells 42(12):821–827. https://doi.org/10.14348/molcells.2019.0298
Acknowledgements
This study received financial support from Dokuz Eylul University (Project Number: 2017.KB.SAG.020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by KUT, BA, ET, and TS, KUT wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This study does not require any ethical statement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tufekci, K.U., Alural, B., Tarakcioglu, E. et al. Lithium inhibits oxidative stress-induced neuronal senescence through miR-34a. Mol Biol Rep 48, 4171–4180 (2021). https://doi.org/10.1007/s11033-021-06430-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06430-w