Abstract
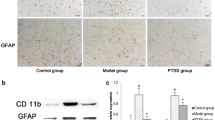
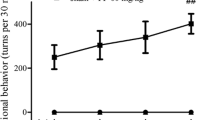
Neuroinflammation is the key factor associated with the progression of Parkinson’s disease (PD). Pramipexole (PPX) has anti-inflammatory and antioxidant properties. This study explored the effects of PPX on PD and its related mechanisms. A PD rat model was established using 6-hydroxydopamine (6-OHDA). Thirty rats were divided into the following three groups: control, PD, and PD + PPX. The rats in the PD and PD + PPX groups were first administered 6-OHDA and then respectively treated with saline and PPX. Afterward, rotational behavior tests were performed to evaluate the efficiency of PPX. The level of tyrosine hydroxylase (TH) was measured using immunohistochemical staining. Subsequently, real-time quantitative PCR (RT-qPCR) and western blot were used to determine the expression of α-synuclein (α-syn), nuclear receptor subfamily 4 group A member 2 (Nurr1), and nuclear factor kappa B (NF-κB). PPX improved the motor behavior of PD rats caused by 6-OHDA. The number of TH-positive neurons in the PD group was significantly lower than that in the control group (P < 0.05), while PPX could rescue 6-OHDA-induced TH loss. RT-qPCR and western blot showed that Nurr1 expression was significantly downregulated in the PD group compared to that of the control group (P < 0.05), while after PPX treatment, its expression was significantly upregulated (P < 0.05). For α-syn and NF-κB, 6-OHDA significantly upregulated their expressions (P < 0.05), whereas PPX reversed them. PPX improved the motor behavior of PD through mediating the inflammatory response and regulating the Nurr1/NF-κB signaling pathway.




Similar content being viewed by others
Data availability
The data in the current study are available from the corresponding authors on reasonable request.
References
Halliday GM, McCann H (2008) Human-based studies on alpha-synuclein deposition and relationship to Parkinson’s disease symptoms. Exp Neurol 209(1):12–21. https://doi.org/10.1016/j.expneurol.2007.07.006
Sabino-Carvalho JL, Samora M, Teixeira AL, Daher M, Vianna LC (2019) Circulatory responses at the onset of handgrip exercise in patients with Parkinson’s disease. Exp Physiol 104(6):793–799
Jankovic J, Chen S, Le WD (2005) The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog Neurobiol 77(1–2):128–138. https://doi.org/10.1016/j.pneurobio.2005.09.001
Allen Reish HE, Standaert DG (2015) Role of alpha-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Park Dis 5(1):1–19. https://doi.org/10.3233/JPD-140491
Figueiredo-Pereira ME, Rockwell P, Schmidt-Glenewinkel T, Serrano P (2014) Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front Mol Neurosci 7:104. https://doi.org/10.3389/fnmol.2014.00104
Russo I, Bubacco L, Greggio E (2014) LRRK2 and neuroinflammation: partners in crime in Parkinson’s disease? J Neuroinflamm 11:52. https://doi.org/10.1186/1742-2094-11-52
Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE (2006) Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6(5):261–281. https://doi.org/10.1016/j.cnr.2006.09.006
Tolosa E, Botta-Orfila T, Morato X, Calatayud C, Ferrer-Lorente R, Marti MJ, Fernandez M, Gaig C, Raya A, Consiglio A, Ezquerra M, Fernandez-Santiago R (2018) MicroRNA alterations in iPSC-derived dopaminergic neurons from Parkinson disease patients. Neurobiol Aging 69:283–291. https://doi.org/10.1016/j.neurobiolaging.2018.05.032
Bassani TB, Vital MA, Rauh LK (2015) Neuroinflammation in the pathophysiology of Parkinson’s disease and therapeutic evidence of anti-inflammatory drugs. Arq Neuropsiquiatr 73(7):616–623. https://doi.org/10.1590/0004-282X20150057
Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, Rupp M, Boroojerdi B (2007) Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol 6(6):513–520. https://doi.org/10.1016/S1474-4422(07)70108-4
Le WD, Jankovic J, Xie W, Appel SH (2000) Antioxidant property of pramipexole independent of dopamine receptor activation in neuroprotection. J Neural Transm (Vienna) 107(10):1165–1173. https://doi.org/10.1007/s007020070030
Piercey MF (1998) Pharmacology of pramipexole, a dopamine D3-preferring agonist useful in treating Parkinson’s disease. Clin Neuropharmacol 21(3):141–151
Hall ED, Andrus PK, Oostveen JA, Althaus JS, VonVoigtlander PF (1996) Neuroprotective effects of the dopamine D2/D3 agonist pramipexole against postischemic or methamphetamine-induced degeneration of nigrostriatal neurons. Brain Res 742(1–2):80–88. https://doi.org/10.1016/s0006-8993(96)00968-7
Wang Y, Yu X, Zhang P, Ma Y, Wang L, Xu H, Sui D (2018) Neuroprotective effects of pramipexole transdermal patch in the MPTP-induced mouse model of Parkinson’s disease. J Pharmacol Sci 138(1):31–37. https://doi.org/10.1016/j.jphs.2018.08.008
Jakaria M, Haque ME, Cho DY, Azam S, Kim IS, Choi DK (2019) Molecular insights into NR4A2(Nurr1): an emerging target for neuroprotective therapy against neuroinflammation and neuronal cell death. Mol Neurobiol 56(8):5799–5814
Chen XX, Qian Y, Wang XP, Tang ZW, Xu JT, Lin H, Yang ZY, Song XB, Lu D, Guo JZ, Bian LG, Li Y, Zhou L, Deng XL (2018) Nurr1 promotes neurogenesis of dopaminergic neuron and represses inflammatory factors in the transwell coculture system of neural stem cells and microglia. CNS Neurosci Ther 24(9):790–800. https://doi.org/10.1111/cns.12825
Shao QH, Yan WF, Zhang Z, Ma KL, Peng SY, Cao YL, Yuan YH, Chen NH (2019) Nurr1: a vital participant in the TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2 cells. Neuropharmacology 144:388–399
Arredondo C, Gonzalez M, Andres ME, Gysling K (2016) Opposite effects of acute and chronic amphetamine on Nurr1 and NF-kappaB p65 in the rat ventral tegmental area. Brain Res 1652:14–20. https://doi.org/10.1016/j.brainres.2016.09.031
Neff L, Zeisel M, Sibilia J, Schöller-Guinard M, Klein JP, Wachsmann D (2001) NF-κB and the MAP kinases/AP-1 pathways are both involved in interleukin-6 and interleukin-8 expression in fibroblast-like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell Microbiol 3(10):703–712
Popichak KA, Hammond SL, Moreno JA, Afzali MF, Backos DS, Slayden RD, Safe S, Tjalkens RB (2018) Compensatory expression of Nur77 and Nurr1 regulates NF-kappaB-dependent inflammatory signaling in astrocytes. Mol Pharmacol 94(4):1174–1186. https://doi.org/10.1124/mol.118.112631
Lee E, Park HR, Ji ST, Lee Y, Lee J (2014) Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-kappaB, ERK, and JNK. J Neurosci Res 92(1):130–139. https://doi.org/10.1002/jnr.23307
Sabolek M, Baumann B, Heinrich M, Meyer AK, Herborg A, Liebau S, Maisel M, Hermann A, Ventz K, Schwarz J, Wirth T, Storch A (2009) Initiation of dopaminergic differentiation of Nurr1(−) mesencephalic precursor cells depends on activation of multiple mitogen-activated protein kinase pathways. Stem Cells 27(8):2009–2021. https://doi.org/10.1002/stem.122
Hernandez-Baltazar D, Zavala-Flores LM, Villanueva-Olivo A (2017) The 6-hydroxydopamine model and Parkinsonian pathophysiology: novel findings in an older model. Neurologia 32(8):533–539. https://doi.org/10.1016/j.nrl.2015.06.011
Nishiyama T, Masuda Y, Izawa T, Ohnuma T, Ogura K, Hiratsuka A (2019) Magnolol protects PC12 cells from hydrogen peroxide or 6-hydroxydopamine induced cytotoxicity. J Toxicol Sci 44(11):753–758. https://doi.org/10.2131/jts.44.753
Shan S, Tian L, Fang R (2019) Chlorogenic acid exerts beneficial effects in 6-hydroxydopamine-induced neurotoxicity by inhibition of endoplasmic reticulum stress. Med Sci Monit Int Med J Exp Clin Res 25:453–459. https://doi.org/10.12659/MSM.911166
Hawlitschka A, Holzmann C, Wree A, Antipova V (2018) Repeated intrastriatal botulinum neurotoxin-A injection in hemiparkinsonian rats increased the beneficial effect on rotational behavior. Toxins (Basel). https://doi.org/10.3390/toxins10090368
Borlongan CV, Randall TS, Cahill DW, Sanberg PR (1995) Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res 676(1):231–234. https://doi.org/10.1016/0006-8993(95)00150-o
Xu G, Ao R, Zhi Z, Jia J, Yu B (2019) miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol 234(7):10205–10217. https://doi.org/10.1002/jcp.27690
Caggiu E, Arru G, Hosseini S, Niegowska M, Sechi G, Zarbo IR, Sechi LA (2019) Inflammation, infectious triggers, and Parkinson’s disease. Front Neurol 10:122. https://doi.org/10.3389/fneur.2019.00122
Kin K, Yasuhara T, Kameda M, Date I (2019) Animal models for Parkinson’s disease research: trends in the 2000s. Int J Mol Sci. https://doi.org/10.3390/ijms20215402
Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5(12):1403–1409. https://doi.org/10.1038/70978
Alonso Canovas A, Luquin Piudo R, Garcia Ruiz-Espiga P, Burguera JA, Campos Arillo V, Castro A, Linazasoro G, Lopez Del Val J, Vela L, Martinez Castrillo JC (2014) Dopaminergic agonists in Parkinson’s disease. Neurologia 29(4):230–241. https://doi.org/10.1016/j.nrl.2011.04.012
Zhang QS, Heng Y, Mou Z, Huang JY, Yuan YH, Chen NH (2017) Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol Sin 38(10):1317–1328. https://doi.org/10.1038/aps.2017.49
Wang X, Yang HA, Wang XN, Du YF (2016) Effect of siRNA-induced silencing of cellular prion protein on tyrosine hydroxylase expression in the substantia nigra of a rat model of Parkinson’s disease. Genet Mol Res. https://doi.org/10.4238/gmr.15027406
Narbute K, Pilipenko V, Pupure J, Dzirkale Z, Jonavice U, Tunaitis V, Kriauciunaite K, Jarmalaviciute A, Jansone B, Klusa V, Pivoriunas A (2019) Intranasal administration of extracellular vesicles derived from human teeth stem cells improves motor symptoms and normalizes tyrosine hydroxylase expression in the substantia nigra and striatum of the 6-hydroxydopamine-treated rats. Stem Cells Transl Med 8(5):490–499. https://doi.org/10.1002/sctm.18-0162
Li Y, Niu M, Zhao A, Kang W, Chen Z, Luo N, Zhou L, Zhu X, Lu L, Liu J (2019) CXCL12 is involved in alpha-synuclein-triggered neuroinflammation of Parkinson’s disease. J Neuroinflamm 16(1):263. https://doi.org/10.1186/s12974-019-1646-6
Atik A, Stewart T, Zhang J (2016) Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol 26(3):410–418. https://doi.org/10.1111/bpa.12370
Volpicelli F, Caiazzo M, Greco D, Consales C, Leone L, Perrone-Capano C, Colucci D’Amato L, di Porzio U (2007) Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J Neurochem 102(2):441–453. https://doi.org/10.1111/j.1471-4159.2007.04494.x
Maguire-Zeiss KA, Federoff HJ (2010) Future directions for immune modulation in neurodegenerative disorders: focus on Parkinson’s disease. J Neural Transm (Vienna) 117(8):1019–1025. https://doi.org/10.1007/s00702-010-0431-6
Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A (2012) Alpha-synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med 4(163):163ra156. https://doi.org/10.1126/scitranslmed.3004676
Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137(1):47–59. https://doi.org/10.1016/j.cell.2009.01.038
Flood PM, Qian L, Peterson LJ, Zhang F, Shi JS, Gao HM, Hong JS (2011) Transcriptional factor NF-kappaB as a target for therapy in Parkinson’s disease. Park Dis 2011:216298. https://doi.org/10.4061/2011/216298
Wang S, He H, Chen L, Zhang W, Zhang X, Chen J (2015) Protective effects of salidroside in the MPTP/MPP(+)-induced model of Parkinson’s disease through ROS-NO-related mitochondrion pathway. Mol Neurobiol 51(2):718–728. https://doi.org/10.1007/s12035-014-8755-0
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number U150322).
Author information
Authors and Affiliations
Contributions
XY, HG, and DW designed the research study. HG, DW, JM, and YW conducted the experiments and acquired the data. SJ conducted the statistical analyses. HG and DW drafted the manuscript. XY obtained the funding and revised the manuscript. All authors have reviewed and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The animal experiments were approved by the Ethics Review Committee for Animal Studies of the First Affiliated Hospital of Xinjiang Medical University (Urumqi, China) and were implemented in accordance with the guidelines of animal welfare and animal experiments (IACUC 20160414-07).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, H., Wang, D., Wang, YL. et al. Pramipexole attenuates 6-OHDA-induced Parkinson’s disease by mediating the Nurr1/NF-κB pathway. Mol Biol Rep 48, 3079–3087 (2021). https://doi.org/10.1007/s11033-021-06343-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06343-8