Abstract
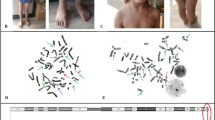
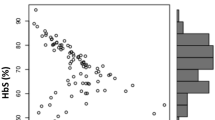
Fanconi anemia (FA) occurs due to genomic instability with predisposition to bone marrow failure, phenotypic abnormalities and cancers. Though mutations in 22 genes leading to DNA repair defect have been identified, the cellular factor such as oxidative stress has also shown to be associated with FA. Nitrosative Stress (NS) is biochemically correlated to many oxidative stress related disorders and the NS as a pathological hallmark in FA has been so far overlooked. We carried out the study first time in Indian patients with FA with an objective to understand the role of NS in the pathogenesis of FA. The study was carried out in 70 FA subjects. The FA subjects were diagnosed by chromosomal breakage analysis. Molecular study was carried out by Next Generation Sequencing and Sanger sequencing. The 3-nitrotyrosine [3-NT] levels were estimated through enzyme-linked immuno-sorbent assay (ELISA) and the nitric oxide synthase genes- NOS1 (c.-420-34221G>A (rs1879417), c.-420-10205C>T (rs499776), c.4286+720G>C (rs81631)) and NOS2 (c.1823C>T (p. Ser608Leu) (rs2297518)) polymorphism were studied by direct sequencing. Chromosomal breakage analysis revealed a high frequency of chromosomal breaks (Mean chromosomal breakage—4.13 ± 1.5 breaks/metaphase) in 70 FA patients as compared to the control. Molecular studies revealed FANCA (58.34%), FANCG (18.34%) and FANCL (16.6%) complementation groups. The 3-nitrotyrosine [3-NT] levels showed to be significantly (p < 0.05) elevated in FA subjects when compared to the age match controls. Genotyping of the NOS2 gene c.1823C>T (p. Ser608Leu) (rs2297518), showed statistically significant (P < 0.05) association with FA. Elevated level of 3-NT is one of the cause of NS and NOS2 gene polymorphism associated with FA is an important target in the treatment regimen.


Similar content being viewed by others
References
Bagby GC (2003) Genetic basis of Fanconi anemia. Curr Opin Hematol 10(1):68–76. http://www.ncbi.nlm.nih.gov/pubmed/12483114. Accessed 24 Jun 2019
Faivre L et al (2000) Association of complementation group and mutation type with clinical outcome in fanconi anemia. European Fanconi Anemia Research Group. Blood 96(13):4064–4070. http://www.ncbi.nlm.nih.gov/pubmed/11110674. Accessed 19 Jun 2019
Mamrak NE, Shimamura A, Howlett NG (2017) Recent discoveries in the molecular pathogenesis of the inherited bone marrow failure syndrome Fanconi anemia. Blood Rev 31(3):93–99. https://doi.org/10.1016/j.blre.2016.10.002
Pagano G, Talamanca AA, Castello G, Pallardó FV, Zatterale A, Degan P (2012) Oxidative stress in Fanconi anaemia: from cells and molecules towards prospects in clinical management. Biol Chem 393(1–2):2012. https://doi.org/10.1515/BC-2011-227
Mastrocola R et al (2005) Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol 187(1):37–44. https://doi.org/10.1677/joe.1.06269
Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H (2004) Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Phtsiol. https://doi.org/10.1152/ajplung.00295.2003.-Nitric
Maines MD (1996) Nitric oxide synthase: characterization and functional analysis. Academic Press, Cambridge
Selleri C, Maciejewski JP (2001) Nitric oxide and cell survival: megakaryocytes say ‘NO.’ J Lab Clin Med 137(4):225–230. https://doi.org/10.1067/mlc.2001.113660
Hadjur S, Jirik FR (2003) Increased sensitivity of Fancc-deficient hematopoietic cells to nitric oxide and evidence that this species mediates growth inhibition by cytokines. Blood J Am Soc Hematol. https://doi.org/10.1182/blood-2002-10-3147
Zhou Q-G, Zhu X-H, Nemes AD, Zhu D-Y (2018) Neuronal nitric oxide synthase and affective disorders. IBRO Rep 5:116–132. https://doi.org/10.1016/j.ibror.2018.11.004
Ryk C, de Verdier P, Montgomery E, Peter-Wiklund N, Wiklund F, Grönberg H (2015) Polymorphisms in the nitric-oxide synthase 2 gene and prostate cancer pathogenesis. Redox Biol 5:419. https://doi.org/10.1016/j.redox.2015.09.029
Shinkai T, Ohmori O, Matsumoto C, Hori H, Kennedy JL, Nakamura J (2004) Genetic association analysis of neuronal nitric oxide synthase gene polymorphism with tardive dyskinesia. NeuroMolecular Med 5(2):163–170. https://doi.org/10.1385/NMM:5:2:163
García-de BT et al (2019) FANCC Dutch founder mutation in a Mennonite family from Tamaulipas. México. Mol Genet Genomic Med 2019:710. https://doi.org/10.1002/mgg3.710
Oostra AB, Nieuwint AWM, Joenje H, de Winter JP (2012) Diagnosis of fanconi anemia: chromosomal breakage analysis. Anemia 2012:1–9. https://doi.org/10.1155/2012/238731
Quick Statistics Calculators (2020) https://www.socscistatistics.com/tests/. Accessed 6 Nov 2020
Du W, Adam Z, Rani R, Zhang X, Pang Q (2008) Forum review oxidative stress in fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal 10(11):1909–1921. https://doi.org/10.1089/ars.2008.2129
El Bassyouni HT (2019) Oxidative stress—a phenotypic hallmark of fanconi anemia and down syndrome: the effect of antioxidants. www.amhsr.org
Jeremy JY, Yim AP, Wan S, Angelini GD (2010) Oxidative stress, nitric oxide, and vascular disease. J Card Surg 17(4):324–327. https://doi.org/10.1111/j.1540-8191.2001.tb01151.x
Morris G, Puri BK, Olive L, Carvalho AF, Berk M, Maes M (2019) Emerging role of innate B1 cells in the pathophysiology of autoimmune and neuroimmune diseases: association with inflammation, oxidative and nitrosative stress and autoimmune responses. Pharmacol Res 148:2019. https://doi.org/10.1016/j.phrs.2019.104408
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15(1):323–350. https://doi.org/10.1146/annurev.immunol.15.1.323
Sabuncuoglu S, Öztas Y, Uçkan-Çetinkaya D, Özgünes N, Özgünes H (2012) Oxidative protein damage with carbonyl levels and nitrotyrosine expression after chemotherapy in bone marrow transplantation patients. Pharmacology 89(5–6):283–286. https://doi.org/10.1159/000337040
Kröncke K (2003) Nitrosative stress and transcription. Biol Chem 384:1365–1377
Shyamsunder P et al (2016) Impaired mitophagy in Fanconi anemia is dependent on mitochondrial fission. Oncotarget 7(36):58065–58074. https://doi.org/10.18632/oncotarget.11161
Sumpter R et al (2016) Fanconi anemia proteins function in mitophagy and immunity. Cell. https://doi.org/10.1016/j.cell.2016.04.006
Hadjur S, Jirik FR (2003) Increased sensitivity of Fancc-deficient hematopoietic cells to nitric oxide and evidence that this species mediates growth inhibition by cytokines. Blood 101(10):3877–3884. https://doi.org/10.1182/blood-2002-10-3147
Zotova EV et al (2005) Polymorphic markers of the NO synthase genes and genetic predisposition to diabetic polyneuropathy in patients with type 1 diabetes mellitus. Mol Biol (Mosk) 39(2):224–229
Tajouri L et al (2004) Investigation of an inducible nitric oxide synthase gene (NOS2A) polymorphism in a multiple sclerosis population. Brain Res Bull 64(1):9–13. https://doi.org/10.1016/j.brainresbull.2004.04.019
Miguel Gómez L et al (2007) A polymorphism in the inducible nitric oxide synthase gene is associated with tuberculosis. Tuberculosis 87(4):288–294. https://doi.org/10.1016/j.tube.2007.03.002
Jorge YC, Duarte MC, Silva AE (2010) Gastric cancer is associated with NOS2 -954G/C polymorphism and environmental factors in a Brazilian population. BMC Gastroenterol 10:2010. https://doi.org/10.1186/1471-230X-10-64
Özhan G et al (2012) Acute pancreatitis is associated with Ser608Leu iNOS polymorphism. Folia Biol (Praha) 58(6):256–260
Acknowledgements
We would like to thank patients for participating in our study. We also thank the paediatricians and hemato-oncologists for the clinical assessment of the FA patients.
Funding
This study was carried out from DST/SERB (Grant no. EEQ/2016/000510; B. R.V).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no conflict of interest.
Ethical approval
The study was approved by Institution Ethics Committee for research on Human subjects of ICMR-National institute of Immunohematology.
Informed consent
Informed written consent was obtained from all individual participants included in the study and in case of minors; informed written assent was taken from the parents.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
George, M., Solanki, A., Mohanty, P. et al. Nitric oxide synthase-2 (NOS2) gene polymorphism c.1832C>T (Ser608Leu) associated with nitrosative stress in Fanconi anaemia. Mol Biol Rep 48, 2519–2525 (2021). https://doi.org/10.1007/s11033-021-06293-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06293-1