Abstract
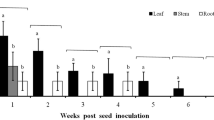
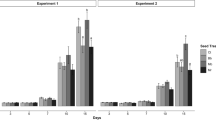
Entomopathogenic fungi are an important factor in the natural regulation of arthropod populations. Moreover, some can exist as an endophyte in many plant species and establish a mutualistic relationship. In this study, we have investigated the endophytic growth of Beauveria bassiana within different tissues of Phaseolus vulgaris in the presence and absence of Tetranuychus urticae. After the colonization of the B. bassiana within the internal tissues of P. vulgaris. The susceptibility of T. urticae appeared to depend on the life stage where high, moderate, and low mortalities were recorded among adults, nymphs, and eggs, respectively. In addition, this study provided, for the first time, molecular insight into the endophytic growth of B. bassiana by analyzing the expression of several genes involved in the development of the entomopathogenic fungi at 0-, 2-, and 7- days post-inoculation. B. bassiana displayed preferential tissue colonization within P. vulgaris that can be put into the following order based on the detection rate: leaf > stem > root. After analyzing the development-implicated genes (degrading enzymes, sugar transporter, hydrophobins, cell wall synthesis, secondary metabolites, stress management), the most remarkable finding is the detection of behavioral change between parasitic and endophytic Beauveria during post-penetration events. This study elucidates the tri-trophic interaction between fungus-plant-herbivore.


Similar content being viewed by others
Data availability
All data are available.
References
Smith GS, O'Day MH, Reid W (2019) Pecan pest management: insects and diseases. Department of entomology, Kansas State University. https://extension2.missouri.edu/mp711. Accessed July 2020
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12
Plant R, Freudenberger D (2005) Changes in global agriculture: a framework for diagnosing ecosystem effects and identifying response options. WWF Macroeconomics Program Office Washington, DC, USA
Faria MR, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43:237–256
Cafarchia C, Immediato D, Iatta R, Ramos RAN, Lia RP, Porretta D, Figueredo LA, Dantas-Torres F, Otranto D (2015) Native strains of Beauveriabassiana for the control of Rhipicephalussanguineus sensu lato. Parasite Vectors 8:80
Chandler D, Davidson G, Jacobson RJ (2005) Laboratory and glasshouse evaluation of entomopathogenic fungi against the two-spotted spider mite, Tetranychusurticae (Acari: Tetranychidae), on tomato, Lycopersicon esculentum. Biocontrol Sci Technol 15:37–54
Immediato D, Camarda A, Iatta R, Puttilli MR, Ramos RAN, Di Paola G, Giangaspero A, Otranto D, Cafarchia C (2015) Laboratory evaluation of a native strain of Beauveriabassiana for controlling Dermanyssusgallinae (De Geer, 1778)(Acari: Dermanyssidae). Vet Parasitol 212:478–482
Kaiser D, Handschin S, Rohr RP, Bacher S, Grabenweger G (2020) Co-formulation of Beauveriabassiana with natural substances to control pollen beetles–synergy between fungal spores and colza oil. Biol Control 140:104106
Morales AD, Castillo A, Cisneros J, Valle JF, Gómez J (2019) Effect of spinosad combined with Beauveriabassiana (Hypocreales: Clavicipitaceae) on Hypothenemushampei (Coleoptera: Curculionidae) under laboratory conditions. J Entomol Sci 54:106–109
Mwamburi LA, Laing MD, Miller RM (2019) External development of the entomopathogenic fungus beauveria bassiana on the housefly (Muscadomestica). Proc Natl Acad Sci India B 89:833–839
Yanar Y, Yanar D, Demir B, Karan YB (2019) Effects of local entomopathogen Beauveriabassiana isolates against Sitophilusgranaries (Coleoptera). Poljoprivreda i Sumarstvo 65:49–55
Feng MG, Poprawski TJ, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveriabassiana for insect control: current status. Biocontrol Sci Technol 4:3–34
Butt TM, Coates CJ, Dubovskiy IM, Ratcliffe NA (2016) Entomopathogenic fungi: new insights into host–pathogen interactions. Anonymous advances in genetics, vol 94. Elsevier, Amsterdam, pp 307–364
Mondal S, Baksi S, Koris A, Vatai G (2016) Journey of enzymes in entomopathogenic fungi. Pac Sci Rev A 18:85–99
Zhang S, Xia YX, Kim B, Keyhani NO (2011) Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveriabassiana. Mol Microbiol 80:811–826
Tartar A, Shapiro AM, Scharf DW, Boucias DG (2005) Differential expression of chitin synthase (CHS) and glucan synthase (FKS) genes correlates with the formation of a modified, thinner cell wall in in vivo-produced Beauveriabassiana cells. Mycopathologia 160:303–314
Xu Y, Orozco R, Wijeratne EK, Espinosa-Artiles P, Gunatilaka AL, Stock SP, Molnár I (2009) Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveriabassiana. Fungal Genet Biol 46:353–364
Xu Y, Orozco R, Wijeratne EK, Gunatilaka AL, Stock SP, Molnár I (2008) Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveriabassiana. Chem Biol 15:898–907
Xiao G, Ying S, Zheng P, Wang Z, Zhang S, Xie X, Shang Y, Leger RJS, Zhao G, Wang C (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveriabassiana. Sci Rep 2:483
Zhang Y, Zhao J, Fang W, Zhang J, Luo Z, Zhang M, Fan Y, Pei Y (2009) Mitogen-activated protein kinase hog1 in the entomopathogenic fungus Beauveriabassiana regulates environmental stress responses and virulence to insects. Appl Environ Microbiol 75:3787–3795
Ramakuwela T, Hatting J, Bock C, Vega FE, Wells L, Mbata GN, Shapiro-Ilan D (2020) Establishment of Beauveriabassiana as a fungal endophyte in pecan (Caryaillinoinensis) seedlings and its virulence against pecan insect pests. Biol Control 140:104102
Ownley BH, Griffin MR, Klingeman WE, Gwinn KD, Moulton JK, Pereira RM (2008) Beauveriabassiana: endophytic colonization and plant disease control. J Invertebr Pathol 98:267–270
Russo ML, Scorsetti AC, Vianna MF, Allegrucci N, Ferreri NA, Cabello MN, Pelizza SA (2019) Effects of endophytic Beauveriabassiana (Ascomycota: Hypocreales) on biological, reproductive parameters and food preference of the soybean pest Helicoverpagelotopoeon. J King Saud Univ Sci 31:1077–1082
Vega FE (2018) The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia 110:4–30
Barra-Bucarei L, France Iglesias A, Gerding González M, Silva Aguayo G, Carrasco-Fernández J, Castro JF, Ortiz Campos J (2020) Antifungal activity of Beauveriabassiana endophyte against botrytis cinerea in two solanaceae crops. Microorganisms 8:65
Afandhi A, Widjayanti T, Emi AAL, Tarno H, Afiyanti M, Handoko RNS (2019) Endophytic fungi Beauveriabassiana Balsamo accelerates growth of common bean (Phaeseolusvulgaris L.). Chem Biol 6:11
Gange AC, Koricheva J, Currie AF, Jaber LR, Vidal S (2019) Meta-analysis of the role of entomopathogenic and unspecialized fungal endophytes as plant bodyguards. New Phytol 223:2002–2010
Vidal S, Jaber LR (2015) Entomopathogenic fungi as endophytes: plant–endophyte–herbivore interactions and prospects for use in biological control. Curr Sci 109:46–54
Jaber LR, Ownley BH (2018) Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol Control 116:36–45
Quesada-Moraga E (2020) Entomopathogenic fungi as endophytes: their broader contribution to IPM and crop production. Biocontrol Sci Technol. https://doi.org/10.1080/09583157.2020.1771279
Quesada-Moraga E, Yousef-Naef M, Garrido-Jurado I (2020) Advances in the use of entomopathogenic fungi as biopesticides in suppressing crop insect pests. In: Nick Birch N, Glare T (eds) Biopesticides for sustainable agriculture. Burleigh Dodds Science Publishing, Cambridge, pp 63–98
González-Mas N, Sánchez-Ortiz A, Valverde-García P, Quesada-Moraga E (2019) Effects of endophytic entomopathogenic ascomycetes on Aphisgossypii glover life-history traits and interaction with melon plants. Insects 10:165
Gurulingappa P, Sword GA, Murdoch G, McGee PA (2010) Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol Control 55:34–41
Akutse KS, Maniania NK, Fiaboe K, Van den Berg J, Ekesi S (2013) Endophytic colonization of Viciafaba and Phaseolusvulgaris (Fabaceae) by fungal pathogens and their effects on the life-history parameters of Liriomyzahuidobrensis (Diptera: Agromyzidae). Fungal Ecol 6:293–301
Behie SW, Zelisko PM, Bidochka MJ (2012) Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 336:1576–1577
Behie SW, Jones SJ, Bidochka MJ (2015) Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol 13:112–119
Wagner BL, Lewis LC (2000) Colonization of corn, Zeamays, by the entomopathogenic fungus Beauveriabassiana. Appl Environ Microbiol 66:3468–3473
Myers JR, Kmiecik K (2017) Common bean: economic importance and relevance to biological science research. Anonymous the common bean genome. Springer, Cham, pp 1–20
Capinera L (2008) Twospotted spider mite, Tetranychusurticae Koch (Acari: Tetranychidae). In: Capinera JL (ed) Encyclopedia of entomology. Springer, Dordrecht
Boubou A, Migeon A, Roderick GK, Navajas M (2011) Recent emergence and worldwide spread of the red tomato spider mite, Tetranychusevansi: genetic variation and multiple cryptic invasions. Biol Invasions 13:81–92
Dagli F, Tunc I (2001) Dicofol resistance in Tetranychus cinnabarinus: resistance and stability of resistance in populations from Antalya, Turkey. Pest Manag Sci 57:609–614
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychusurticae and other important Acari: a review. Insect Biochem Mol 40:563–572
Goettel MS, Inglis GD (1997) Fungi: hyphomycetes. Manual of techniques in insect pathology. Elsevier, Amsterdam, pp 213–249
Posada F, Aime MC, Peterson SW, Rehner SA, Vega FE (2007) Inoculation of coffee plants with the fungal entomopathogen Beauveriabassiana (Ascomycota: Hypocreales). Mycol Res 111:748–757
Rehner SA, Minnis AM, Sung G, Luangsa-ard JJ, Devotto L, Humber RA (2011) Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 103:1055–1073
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols 18:315–322
Landa BB, López-Díaz C, Jiménez-Fernández D, Montes-Borrego M, Muñoz-Ledesma FJ, Ortiz-Urquiza A, Quesada-Moraga E (2013) In-planta detection and monitorization of endophytic colonization by a Beauveriabassiana strain using a new-developed nested and quantitative PCR-based assay and confocal laser scanning microscopy. J Invertebr Pathol 114:128–138
Batta YA (2018) Efficacy of two species of entomopathogenic fungi against the stored-grain pest, Sitophilusgranarius L. (Curculionidae: Coleoptera), via oral ingestion. Egypt J Biol Pest Control 28:44–52
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Zhou Y, Zhang Y, Luo Z, Fan Y, Tang G, Liu L, Pei Y (2012) Selection of optimal reference genes for expression analysis in the entomopathogenic fungus Beauveriabassiana during development, under changing nutrient conditions, and after exposure to abiotic stresses. Appl Microbiol Biotechnol 93:679–685
Fang W, Feng J, Fan Y, Zhang Y, Bidochka MJ, Leger RJS, Pei Y (2009) Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveriabassiana. J Invertebr Pathol 102:155–159
Fang W, Leng B, Xiao Y, Jin K, Ma J, Fan Y, Feng J, Yang X, Zhang Y, Pei Y (2005) Cloning of Beauveriabassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl Environ Microbiol 71:363–370
Abdo C, Nemer N, Nemer G, Jawdah YA, Atamian H, Kawar NS (2008) Isolation of Beauveria species from Lebanon and evaluation of its efficacy against the cedar web-spinning sawfly, Cephalciatannourinensis. Biocontrol 53:341–352
Parsa S, García-Lemos AM, Castillo K, Ortiz V, López-Lavalle LAB, Braun J, Vega FE (2016) Fungal endophytes in germinated seeds of the common bean, Phaseolusvulgaris. Fungal Biol 120:783–790
Parsa S, Ortiz V, Vega FE (2013) Establishing fungal entomopathogens as endophytes: towards endophytic biological control. J Vis Exp. https://doi.org/10.3791/50360
McKinnon AC, Saari S, Moran-Diez ME, Meyling NV, Raad M, Glare TR (2017) Beauveriabassiana as an endophyte: a critical review on associated methodology and biocontrol potential. Biocontrol 62:1–17
Healey A, Furtado A, Cooper T, Henry RJ (2014) Protocol: a simple method for extracting next-generation sequencing quality genomic DNA from recalcitrant plant species. Plant Methods 10:21
Al Khoury C, Guillot J, Nemer N (2020) Susceptibility and development of resistance of the mite Tetranychusurticae to aerial conidia and blastospores of the entomopathogenic fungus Beauveriabassiana. Syst App Acarol 25:429–443
Tehri K, Gulati R, Geroh M, Dhankhar SK (2015) Dry weather: a crucial constraint in the field efficacy of entomopathogenic fungus BeauveriaBassiana against Tetranychusurticae Koch (Acari: Tetranychidae). J Entomol 3:287–291
Ortucu S, Algur OF (2017) A laboratory assessment of two local strains of the Beauveriabassiana (Bals.) Vuill. against the Tetranychusurticae (acari: Tetranychidae) and their potential as a mycopesticide. J Pathog 2107:1–7
Gómez-Vidal S, Lopez-Llorca LV, Jansson H, Salinas J (2006) Endophytic colonization of date palm (Phoenixdactylifera L.) leaves by entomopathogenic fungi. Micron 37:624–632
Quesada-Moraga E, Ruiz-García A, Santiago-Alvarez C (2006) Laboratory evaluation of entomopathogenic fungi Beauveriabassiana and Metarhiziumanisopliae against puparia and adults of Ceratitiscapitata (Diptera: Tephritidae). J Econ Entomol 99:1955–1966
Bensoussan N, Zhurov V, Yamakawa S, O’Neil CH, Suzuki T, Grbić M, Grbić V (2018) The digestive system of the two-spotted spider mite, Tetranychusurticae Koch, in the context of the mite-plant interaction. Front Plant Sci 9:1206
Charnley AK, Leger RS (1991) The role of cuticle-degrading enzymes in fungal pathogenesis in insects. In: Ann R (ed) The fungal spore and disease initiation in plants and animals. Springer, Boston, p 267
Mannino MC, Huarte-Bonnet C, Davyt-Colo B, Pedrini N (2019) Is the insect cuticle the only entry gate for fungal infection? insights into alternative modes of action of entomopathogenic fungi. J Fungi 5:33
Wan J, Zhang X, Neece D, Ramonell KM, Clough S, Kim S, Stacey MG, Stacey G (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20:471–481
Behie SW, Moreira CC, Sementchoukova I, Barelli L, Zelisko PM, Bidochka MJ (2017) Carbon translocation from a plant to an insect-pathogenic endophytic fungus. Nat Commun 8:1–5
Branine M, Bazzicalupo A, Branco S (2019) Biology and applications of endophytic insect-pathogenic fungi. PLoS Pathog 15:e1007831
Holder DJ, Kirkland BH, Lewis MW, Keyhani NO (2007) Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology 153:3448–3457
Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA (2014) Induced systemic resistance by beneficial microbes. Ann Rev Phytopathol 52:347–375
Wu Q, Patocka J, Nepovimova E, Kuca K (2018) A review on the synthesis and bioactivity aspects of beauvericin, a fusarium mycotoxin. Front Pharmacol 9:1338
Barelli L, Moonjely S, Behie SW, Bidochka MJ (2016) Fungi with multifunctional lifestyles: endophytic insect pathogenic fungi. Plant Mol Biol 90:657–664
Zimmermann G (2007) Review on safety of the entomopathogenic fungi Beauveriabassiana and Beauveriabrongniartii. Biocontrol Sci Technol 17:553–596
Strasser H, Vey A, Butt TM (2000) Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci Technol 10:717–735
Mascarin GM, Jaronski ST (2016) The production and uses of Beauveriabassiana as a microbial insecticide. World J Microbiol Biotechnol 32:177
Funding
This work was funded by the Lebanese National Council for Scientific Research (CNRS), grant name (CNRS-L/USEK) and “Coopération pour l'évaluation et le développement de la recherche” CEDRE grant number 37349SA.
Author information
Authors and Affiliations
Contributions
The study conception and design, material preparation, data collection and analysis were performed by Charbel Al Khoury.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Khoury, C. Molecular insight into the endophytic growth of Beauveria bassiana within Phaseolus vulgaris in the presence or absence of Tetranychus urticae. Mol Biol Rep 48, 2485–2496 (2021). https://doi.org/10.1007/s11033-021-06283-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06283-3