Abstract
Background
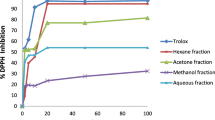
The pawpaw tree has several beneficial effects. However, no studies have been conducted to address the mechanisms underlying the cytotoxic effects of pawpaw extracts against cancer cells, and no study has investigated the anti-inflammatory effects. Hence, in this study, the growth-inhibitory effects of pawpaw (Asimina triloba [L.] Dunal) extracts against gastric (AGS) and cervical (HeLa) cancer cells and the inhibitory effects of pawpaw extracts against inflammatory factors (NO, TNF-α, IL-6, iNOS, and COX-2) were investigated.
Methods and results
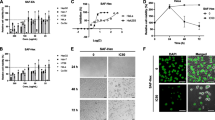
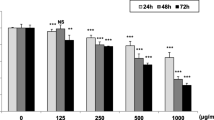
The viability of AGS and HeLa cells, the analysis of cell cycle, and the expression of apoptosis marker protein were determined using MTT assay, FACS, western blotting, and TUNEL assays. The inflammatory factors were determined using Griess method, ELISA assay kit, and RAW 264.7 cells. The IC50 values of twig and unripe fruit extracts for AGS cells were 82.01 and 100.61 µg/mL, respectively. For HeLa cells, pawpaw twig extracts exhibited the strongest ability to inhibit cervical cancer cell growth (IC50 = 97.73 µg/mL). Analysis of the cell cycle phase distribution and expression of the apoptosis regulatory proteins BCL-2, BAX, caspase-3, and PARP showed that pawpaw twig, root, and unripe fruit extracts induced Sub G1 cell cycle arrest and apoptosis of AGS and HeLa cells. In addition, the twig, root, and unripe fruit extracts of pawpaw effectively inhibited the inflammatory makers NO, TNF-α, IL-6, and iNOS. Particularly, the twig, root, and unripe fruit extracts at concentrations of 50 µg/mL exhibited > 50% inhibition of TNF-α.
Conclusions
These findings indicate that pawpaw extracts are natural therapeutic agents that may be used for the prevention and treatment of gastric and cervical cancers, and encourage further studies on the anti-inflammatory potential of the pawpaw tree.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Feigin V (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet 388:1459–1544. https://doi.org/10.1016/S0140-6736(16)31012-1
Wild CP, Stewart BW (2014) World Cancer Report 2014. World Health Organization, Lyon, France
Han HH, Park JW, Na JC, Chung BH, Kim CS, Ko WJ (2015) Epidemiology of prostate cancer in South Korea. Prostate Int 3(3):99–102. https://doi.org/10.1016/j.prnil.2015.06.003
Kim JH, Park MK (2010) Effects of preventive sexual education of HPV on HPV knowledge, cervical cancer preventive behaviors, and sexual autonomy in female university students. J Korean Acad Nurs 16(2):257–264. https://doi.org/10.5977/jkasne.2010.16.2.257
Kim EJ, Kim GT, Kim BM, Lim EG, Kim SY, Kim YM (2016) Apoptosis-induced effects of extract from Artemisia annua Linné by modulating Akt/mTOR/GSK-3β signal pathway in AGS human gastric carcinoma cells. J Korean Soc Food Sci Nutr 45(9):1257–1264. https://doi.org/10.3746/jkfn.2016.45.9.1257
Kasibhatla S, Tseng B (2003) Why target apoptosis in cancer treatment? Mol Cancer Ther 2(6):573–580
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257. https://doi.org/10.3736/jintegrmed2013036
Hseu YC, Wu FY, Wu JJ, Chen JY, Chang WH, Lu FJ, Lai YC, Yang HL (2005) Anti-inflammatory potential of Antrodia camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-κB pathway. Int Immunopharmacol 5(13):1914–1925. https://doi.org/10.1016/j.intimp.2005.06.013
Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A (1993) Enhand secretion of tumour necrosis factor-alpha, IL-6, and IL-1β by isolated lamina ropria monouclear cells from patients with ulcretive cilitis and Crohn’s disease. Clin Exp Immunol 94(1):174–181. https://doi.org/10.1111/j.1365-2249.1993.tb05997.x
Umesalma S, Sudhandiran G (2010) Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol 107(2):650–655. https://doi.org/10.1111/j.1742-7843.2010.00565.x
Callaway MB (1993) Pawpaw (Asimina triloba). A “tropical” fruit for temperate climates. Wiley, New York, pp 505–515
Brannan RG, Peters T, Talcott ST (2015) Phytochemical analysis of ten varieties of pawpaw (Asimina triloba [L.] Dunal) fruit pulp. Food Chem 168:656–661. https://doi.org/10.1016/j.foodchem.2014.07.018
Nam JS, Jang HL, Rhee YH (2017) Antioxidant activities and phenolic compounds of several tissues of pawpaw (Asimina triloba [L.] Dunal) grown in Korea. J Food Sci 82(8):1827–1833. https://doi.org/10.1111/1750-3841.13806
Brannan RG, Salabak DE (2009) Ability of methanolic seed extracts of pawpaw (Asimina triloba) to inhibit n-3 fatty acid oxidation initiated by peroxyl radicals and reactive oxygen, nitrogen, and sulfur. Food Chem 114(2):453–458. https://doi.org/10.1016/j.foodchem.2008.09.071
Coothankandaswamy V, Liu Y, Mao SC, Morgan JB, Mahdi F, Jekabsons MB, Nagle DG, Zhou YD (2010) The alternative medicine pawpaw and its acetogenin constituents suppress tumor angiogenesis via the HIF-1/VEGF pathway. J Nat Prod 73(5):956–961. https://doi.org/10.1021/np100228d
Farag MA (2009) Chemical composition and biological activities of Asimina triloba leaf essential oil. Pharm Biol 47(10):982–986. https://doi.org/10.1080/13880200902967995
Harris GG, Brannan RG (2009) A preliminary evaluation of antioxidant compounds, reducing potential, and radical scavenging of pawpaw (Asimina tribloba) fruit pulp from different stages of ripeness. LWT-Food Sci Technol 42(1):275–279. https://doi.org/10.1016/j.lwt.2008.05.006
Kobayashi H, Wang C, Pomper KW (2008) Phenolic content and antioxidant capacity of pawpaw fruit (Asimina triloba L.) at different ripening stages. HortScience 43(1):268–270. https://doi.org/10.21273/HORTSCI.43.1.268
Woo MH, Cho KY, Zhang Y, Zeng L, Gu ZM, McLaughlin JL (1995) Asimilobin and cis-and trans-murisolinones, novel bioactive annonaceous acetogenins from the seeds of Asimina triloba. J Nat Prod 58(10):1533–1542. https://doi.org/10.1021/np50124a009
Zhao GX, Miesbauer LR, Smith DL, McLaughlin JL (1994) Asimin, asiminacin, and asiminecin: Novel highly cytotoxic asimicin isomers from Asimina triloba. J Med Chem 37(13):1971–1976. https://doi.org/10.1021/jm00039a009
Nam JS, Park SY, Lee HJ, Lee SO, Jang HL, Rhee YH (2018) Correlation between acetogenin content and antiproliferative activity of pawpaw (Asimina triloba [L.] Dunal) fruit pulp grown in Korea. J Food Sci. 83(5):1430–1435. https://doi.org/10.1111/1750-3841.14144
Ko YM, Wu TY, Wu YC, Chang FR, Guh JY, Chuang LY (2011) Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells. J Ethnopharmacol 137(3):1283–1290. https://doi.org/10.1016/j.jep.2011.07.056
Kim H, Moon JY, Kim H, Lee DS, Cho M, Choi HK, Kim YS, Mosaddik A, Cho SK (2010) Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem 121(2):429–436. https://doi.org/10.1016/j.foodchem.2009.12.060
Akter R, Uddin SJ, Grice ID, Tiralongo E (2014) Cytotoxic activity screening of Bangladeshi medicinal plant extracts. J Nat Med 68(1):246–252. https://doi.org/10.1007/s11418-013-0789-5
Choi SR, You DH, Ahn MS, Song EJ, Seo SY, Choi MK, Kim YS, Kim MK, Choi DG (2012) Cytotoxicity of methanol extract from Cudrania tricuspidata bureau. Korean J Med Crop Sci 20(3):153–158. https://doi.org/10.7783/kjmcs.2012.20.3.153
Hashemi L, Asadi-Samani M, Moradi MT, Alidadi S (2017) Anticancer activity and phenolic compounds of Pistacia atlantica extract. Int J Pharm Phytopharm Res 7(2):26–31. https://doi.org/10.24896/eijppr.2017725
Saralamma VVG, Nagappan A, Hong GE, Lee HJ, Yumnam S, Raha S, Heo JD, Lee SJ, Lee WS, Kim EH, Kim GS (2015) Poncirin induces apoptosis in AGS human gastric cancer cells through extrinsic apoptotic pathway by up-regulation of Fas ligand. Int J Mol Sci 16(9):22676–22691. https://doi.org/10.3390/ijms160922676
Mane SD, Thoh M, Sharma D, Sandur SK, Naidu KA (2016) Ascorbyl stearate promotes apoptosis through intrinsic mitochondrial pathway in HeLa cancer cells. Anticancer Res 36(12):6409–6417. https://doi.org/10.21873/anticanres.11238
González-Montoya M, Hernández-Ledesma B, Silván JM, Mora-Escobedo R, Martínez-Villaluenga C (2018) Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem 242:75–82. https://doi.org/10.1016/j.foodchem.2017.09.035
Xu J, Zhao Y, Aisa HA (2017) Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264. 7 macrophages. Pharm Biol 55(1):2095–2101. https://doi.org/10.1080/13880209.2017.1357737
Karki R, Park CH, Kim DW (2013) Extract of buckwheat sprouts scavenges oxidation and inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages (RAW264. 7). J Integr Med 11(4):246–252. https://doi.org/10.3736/jintegrmed2013036
Bloodsworth A, O’Donnell VB, Freeman BA (2000) Nitric oxide regulation of free radical-and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler Thromb Vasc Biol 20(7):1707–1715. https://doi.org/10.1161/01.ATV.20.7.1707
Liu P, Li H, Luan R, Huang G, Liu Y, Wang M, Chao Q, Wang L, Li D, Fan H, Chen D, Li L, Matsuzaki K, Koike K, Zhao F (2019) Identification of β-carboline and canthinone alkaloids as anti-inflammatory agents but with different inhibitory profile on the expression of iNOS and COX-2 in lipopolysaccharide-activated RAW 264.7 macrophages. J Nat Med. 73(1):124–130. https://doi.org/10.1007/s11418-018-1251-5
Abbaszadeh H, Keikhaei B, Mottaghi S (2019) A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother Res 33(8):2002–2014. https://doi.org/10.1002/ptr.6403
Zhu F, Du B, Xu B (2018) Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit Rev Food Sci Nutr 58(8):1260–1270. https://doi.org/10.1080/10408398.2016.1251390
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. https://doi.org/10.1038/nature07205
Acknowledgements
Not applicable.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
J-SN performed the study and wrote the manuscript, S-YP, S-OL and H-JL helped experiment and data arrangement, H-LJ and YHR planed the study, analyzed the results, and aided writing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This paper does not contain any studies with animals or human participants. Therefore no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nam, JS., Park, SY., Lee, SO. et al. The growth-inhibitory effects of pawpaw (Asimina triloba [L.] Dunal) roots, twigs, leaves, and fruit against human gastric (AGS) and cervical (HeLa) cancer cells and their anti-inflammatory activities. Mol Biol Rep 48, 2173–2181 (2021). https://doi.org/10.1007/s11033-021-06226-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06226-y