Abstract
Mutations in nuclear-encoded protein subunits of the mitochondrial ribosome are an increasingly recognised cause of oxidative phosphorylation system (OXPHOS) disorders. Among them, mutations in the MRPL44 gene, encoding a structural protein of the large subunit of the mitochondrial ribosome, have been identified in four patients with OXPHOS defects and early-onset hypertrophic cardiomyopathy with or without additional clinical features. A 23-year-old individual with cardiac and skeletal myopathy, neurological involvement, and combined deficiency of OXPHOS complexes in skeletal muscle was clinically and genetically investigated. Analysis of whole-exome sequencing data revealed a homozygous mutation in MRPL44 (c.467 T > G), which was not present in the biological father, and a region of homozygosity involving most of chromosome 2, raising the possibility of uniparental disomy. Short-tandem repeat and genome-wide SNP microarray analyses of the family trio confirmed complete maternal uniparental isodisomy of chromosome 2. Mitochondrial ribosome assembly and mitochondrial translation were assessed in patient derived-fibroblasts. These studies confirmed that c.467 T > G affects the stability or assembly of the large subunit of the mitochondrial ribosome, leading to impaired mitochondrial protein synthesis and decreased levels of multiple OXPHOS components. This study provides evidence of complete maternal uniparental isodisomy of chromosome 2 in a patient with MRPL44-related disease, and confirms that MRLP44 mutations cause a mitochondrial translation defect that may present as a multisystem disorder with neurological involvement.




Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information.
References
Pearce S, Nezich CL, Spinazzola A (2013) Mitochondrial diseases: translation matters. Mol Cell Neurosci 55:1–12. https://doi.org/10.1016/j.mcn.2012.08.013
Amunts A, Brown A, Toots J, Scheres SHW, Ramakrishnan V (2015) Ribosome. The structure of the human mitochondrial ribosome. Science 348(6):95–98. https://doi.org/10.1126/science.aaa1193
Rorbach J, Gao F, Powell CA, D’Souza A, Lightowlers RN, Minczuk M, Chrzanowska-Lightowlers ZM (2016) Human mitochondrial ribosomes can switch their structural RNA composition. Proc Natl Acad Sci USA 113(43):12198–12201. https://doi.org/10.1073/pnas.1609338113
Boczonadi V, Horvath R (2014) Mitochondria: impaired mitochondrial translation in human disease. Int J Biochem Cell Biol 48:77–84. https://doi.org/10.1016/j.biocel.2013.12.011
Miller C, Saada A, Shaul N, Shabtai N, Ben-Shalom E, Shaag A, Hershkovitz E, Elpeleg O (2004) Defective mitochondrial translation caused by a ribosomal protein (MRPS16) mutation. Ann Neurol 56(5):734–738. https://doi.org/10.1002/ana.20282
Saada A, Shaag A, Arnon S, Dolfin T, Miller C, Fuchs-Telem D, Lombes A, Elpeleg O (2007) Antenatal mitochondrial disease caused by mitochondrial ribosomal protein (MRPS22) mutation. J Med Genet 44(12):784–786. https://doi.org/10.1136/jmg.2007.053116
Galmiche L, Serre V, Beinat M, Assouline Z, Lebre AS, Chretien D, Nietschke P, Benes V, Boddaert N, Sidi D, Brunelle F, Rio M, Munnich A, Rotig A (2011) Exome sequencing identifies MRPL3 mutation in mitochondrial cardiomyopathy. Hum Mutat 32(11):1225–1231. https://doi.org/10.1002/humu.21562
Smits P, Saada A, Wortmann SB, Heister AJ, Brink M, Pfundt R, Miller C, Haas D, Hantschmann R, Rodenburg RJ, Smeitink JA, van den Heuvel LP (2011) Mutation in mitochondrial ribosomal protein MRPS22 leads to Cornelia de Lange-like phenotype, brain abnormalities and hypertrophic cardiomyopathy. Eur J Hum Genet 19(4):394–399. https://doi.org/10.1038/ejhg.2010.214
Carroll CJ, Isohanni P, Poyhonen R, Euro L, Richter U, Brilhante V, Gotz A, Lahtinen T, Paetau A, Pihko H, Battersby BJ, Tyynismaa H, Suomalainen A (2013) Whole-exome sequencing identifies a mutation in the mitochondrial ribosome protein MRPL44 to underlie mitochondrial infantile cardiomyopathy. J Med Genet 50(3):151–159. https://doi.org/10.1136/jmedgenet-2012-101375
Serre V, Rozanska A, Beinat M, Chretien D, Boddaert N, Munnich A, Rotig A (1832) Chrzanowska-Lightowlers ZM (2013) Mutations in mitochondrial ribosomal protein MRPL12 leads to growth retardation, neurological deterioration and mitochondrial translation deficiency. Biochim Biophys Acta 8:1304–1312. https://doi.org/10.1016/j.bbadis.2013.04.014
Baertling F, Haack TB, Rodenburg RJ, Schaper J, Seibt A, Strom TM, Meitinger T, Mayatepek E, Hadzik B, Selcan G, Prokisch H, Distelmaier F (2015) MRPS22 mutation causes fatal neonatal lactic acidosis with brain and heart abnormalities. Neurogenetics 16(3):237–240. https://doi.org/10.1007/s10048-015-0440-6
Distelmaier F, Haack TB, Catarino CB, Gallenmuller C, Rodenburg RJ, Strom TM, Baertling F, Meitinger T, Mayatepek E, Prokisch H, Klopstock T (2015) MRPL44 mutations cause a slowly progressive multisystem disease with childhood-onset hypertrophic cardiomyopathy. Neurogenetics 16(4):319–323. https://doi.org/10.1007/s10048-015-0444-2
Menezes MJ, Guo Y, Zhang J, Riley LG, Cooper ST, Thorburn DR, Li J, Dong D, Li Z, Glessner J, Davis RL, Sue CM, Alexander SI, Arbuckle S, Kirwan P, Keating BJ, Xu X, Hakonarson H, Christodoulou J (2015) Mutation in mitochondrial ribosomal protein S7 (MRPS7) causes congenital sensorineural deafness, progressive hepatic and renal failure and lactic acidemia. Hum Mol Genet 24(8):2297–2307. https://doi.org/10.1093/hmg/ddu747
Kohda M, Tokuzawa Y, Kishita Y, Nyuzuki H, Moriyama Y, Mizuno Y, Hirata T, Yatsuka Y, Yamashita-Sugahara Y, Nakachi Y, Kato H, Okuda A, Tamaru S, Borna NN, Banshoya K, Aigaki T, Sato-Miyata Y, Ohnuma K, Suzuki T, Nagao A, Maehata H, Matsuda F, Higasa K, Nagasaki M, Yasuda J, Yamamoto M, Fushimi T, Shimura M, Kaiho-Ichimoto K, Harashima H, Yamazaki T, Mori M, Murayama K, Ohtake A, Okazaki Y (2016) A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet 12(1):e1005679. https://doi.org/10.1371/journal.pgen.1005679
Kilic M, Oguz KK, Kilic E, Yuksel D, Demirci H, Sagiroglu MS, Yucel-Yilmaz D, Ozgul RK (2017) A patient with mitochondrial disorder due to a novel mutation in MRPS22. Metab Brain Dis 32(5):1389–1393. https://doi.org/10.1007/s11011-017-0074-5
Lake NJ, Webb BD, Stroud DA, Richman TR, Ruzzenente B, Compton AG, Mountford HS, Pulman J, Zangarelli C, Rio M, Boddaert N, Assouline Z, Sherpa MD, Schadt EE, Houten SM, Byrnes J, McCormick EM, Zolkipli-Cunningham Z, Haude K, Zhang Z, Retterer K, Bai R, Calvo SE, Mootha VK, Christodoulou J, Rotig A, Filipovska A, Cristian I, Falk MJ, Metodiev MD, Thorburn DR (2017) Biallelic mutations in MRPS34 lead to instability of the small mitoribosomal subunit and Leigh syndrome. Am J Hum Genet 101(2):239–254. https://doi.org/10.1016/j.ajhg.2017.07.005
Chen A, Tiosano D, Guran T, Baris HN, Bayram Y, Mory A, Shapiro-Kulnane L, Hodges CA, Coban Akdemir Z, Turan S, Jhangiani SN, van den Akker F, Hoppel CL, Salz HK, Lupski JR, Buchner DA (2018) Mutations in the mitochondrial ribosomal protein MRPS22 lead to primary ovarian insufficiency. Hum Mol Genet 27(11):1913–1926. https://doi.org/10.1093/hmg/ddy098
Gardeitchik T, Mohamed M, Ruzzenente B, Karall D, Guerrero-Castillo S, Dalloyaux D, van den Brand M, van Kraaij S, van Asbeck E, Assouline Z, Rio M, de Lonlay P, Scholl-Buergi S, Wolthuis D, Hoischen A, Rodenburg RJ, Sperl W, Urban Z, Brandt U, Mayr JA, Wong S, de Brouwer APM, Nijtmans L, Munnich A, Rotig A, Wevers RA, Metodiev MD, Morava E (2018) Bi-allelic mutations in the mitochondrial ribosomal protein MRPS2 cause sensorineural hearing loss, hypoglycemia, and multiple OXPHOS complex deficiencies. Am J Hum Genet 102(4):685–695. https://doi.org/10.1016/j.ajhg.2018.02.012
Bugiardini E, Mitchell AL, Rosa ID, Horning-Do HT, Pitmann AM, Poole OV, Holton JL, Shah S, Woodward C, Hargreaves I, Quinlivan R, Amunts A, Wiesner RJ, Houlden H, Holt IJ, Hanna MG, Pitceathly RDS, Spinazzola A (2019) MRPS25 mutations impair mitochondrial translation and cause encephalomyopathy. Hum Mol Genet 28(16):2711–2719. https://doi.org/10.1093/hmg/ddz093
Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Needham SJ, Greaves LC, Kirkwood TB, Turnbull DM (2003) Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest 112(9):1351–1360. https://doi.org/10.1172/JCI19435
Pfeffer G, Gorman GS, Griffin H, Kurzawa-Akanbi M, Blakely EL, Wilson I, Sitarz K, Moore D, Murphy JL, Alston CL, Pyle A, Coxhead J, Payne B, Gorrie GH, Longman C, Hadjivassiliou M, McConville J, Dick D, Imam I, Hilton D, Norwood F, Baker MR, Jaiser SR, Yu-Wai-Man P, Farrell M, McCarthy A, Lynch T, McFarland R, Schaefer AM, Turnbull DM, Horvath R, Taylor RW, Chinnery PF (2014) Mutations in the SPG7 gene cause chronic progressive external ophthalmoplegia through disordered mitochondrial DNA maintenance. Brain 137(Pt 5):1323–1336. https://doi.org/10.1093/brain/awu060
Kirby DM, Thorburn DR, Turnbull DM, Taylor RW (2007) Biochemical assays of respiratory chain complex activity. Methods Cell Biol 80:93–119. https://doi.org/10.1016/S0091-679X(06)80004-X
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164. https://doi.org/10.1093/nar/gkq603
Görmez Z, Bakir-Gungor B, Sagiroglu MS (2014) HomSI: a homozygous stretch identifier from next-generation sequencing data. Bioinformatics 30(3):445–447. https://doi.org/10.1093/bioinformatics/btt686
Horga A, Tomaselli PJ, Gonzalez MA, Laura M, Muntoni F, Manzur AY, Hanna MG, Blake JC, Houlden H, Zuchner S, Reilly MM (2016) SIGMAR1 mutation associated with autosomal recessive silver-like syndrome. Neurology 87(15):1607–1612. https://doi.org/10.1212/WNL.0000000000003212
Durigon R, Mitchell AL, Jones AW, Manole A, Mennuni M, Hirst EM, Houlden H, Maragni G, Lattante S, Doronzio PN, Dalla Rosa I, Zollino M, Holt IJ, Spinazzola A (2018) LETM1 couples mitochondrial DNA metabolism and nutrient preference. EMBO Mol Med. https://doi.org/10.15252/emmm.201708550
Calvo SE, Clauser KR, Mootha VK (2016) MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 44(1):1251–1257. https://doi.org/10.1093/nar/gkv1003
Genomics England (2017) The 100,000 genomes project protocol v2. Genomics England, London
Robinson WP (2000) Mechanisms leading to uniparental disomy and their clinical consequences. BioEssays 22(5):452–459. https://doi.org/10.1002/(SICI)1521-1878(200005)22:5%3c452::AID-BIES7%3e3.0.CO;2-K
Liehr T (2014) Uniparental disomny (UPD) in clinical genetics. Springer, Berlin. https://doi.org/10.1007/978-3-642-55288-5
King JE, Dexter A, Gadi I, Zvereff V, Martin M, Bloom M, Vanderver A, Pizzino A, Schmidt JL (2014) Maternal uniparental isodisomy causing autosomal recessive GM1 gangliosidosis: a clinical report. J Genet Couns 23(5):734–741. https://doi.org/10.1007/s10897-014-9720-9
Miyatake S, Tada H, Moriya S, Takanashi J, Hirano Y, Hayashi M, Oya Y, Nakashima M, Tsurusaki Y, Miyake N, Matsumoto N, Saitsu H (2015) Atypical giant axonal neuropathy arising from a homozygous mutation by uniparental isodisomy. Clin Genet 87(4):395–397. https://doi.org/10.1111/cge.12455
Bis DM, Schule R, Reichbauer J, Synofzik M, Rattay TW, Soehn A, de Jonghe P, Schols L, Zuchner S (2017) Uniparental disomy determined by whole-exome sequencing in a spectrum of rare motoneuron diseases and ataxias. Mol Genet Genomic Med 5(3):280–286. https://doi.org/10.1002/mgg3.285
Douglas GV, Wiszniewska J, Lipson MH, Witt DR, McDowell T, Sifry-Platt M, Hirano M, Craigen WJ, Wong LJ (2011) Detection of uniparental isodisomy in autosomal recessive mitochondrial DNA depletion syndrome by high-density SNP array analysis. J Hum Genet 56(12):834–839. https://doi.org/10.1038/jhg.2011.112
Tucker T, Schlade-Bartusiak K, Eydoux P, Nelson TN, Brown L (2012) Uniparental disomy: can SNP array data be used for diagnosis? Genet Med 14:753–756. https://doi.org/10.1038/gim.2012.35
Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, Ward P, Braxton A, Wang M, Buhay C, Veeraraghavan N, Hawes A, Chiang T, Leduc M, Beuten J, Zhang J, He W, Scull J, Willis A, Landsverk M, Craigen WJ, Bekheirnia MR, Stray-Pedersen A, Liu P, Wen S, Alcaraz W, Cui H, Walkiewicz M, Reid J, Bainbridge M, Patel A, Boerwinkle E, Beaudet AL, Lupski JR, Plon SE, Gibbs RA, Eng CM (2014) Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 312(18):1870–1879. https://doi.org/10.1001/jama.2014.14601
Haudry C, de Lonlay P, Malan V, Bole-Feysot C, Assouline Z, Pruvost S, Brassier A, Bonnefont JP, Munnich A, Rotig A, Lebre AS (2012) Maternal uniparental disomy of chromosome 2 in a patient with a DGUOK mutation associated with hepatocerebral mitochondrial DNA depletion syndrome. Mol Genet Metab 107(4):700–704. https://doi.org/10.1016/j.ymgme.2012.10.008
Pei YF, Hu WZ, Hai R, Wang XY, Ran S, Lin Y, Shen H, Tian Q, Consortium GE-s, Consortium A, Consortium UK, Lei SF, Zhang YH, Papasian CJ, Deng HW, Zhang L (2016) Genome-wide association meta-analyses identified 1q43 and 2q32X2 for hip Ward’s triangle areal bone mineral density. Bone 91(1):10. https://doi.org/10.1016/j.bone.2016.07.004
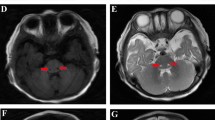
Wray SH, Provenzale JM, Johns DR, Thulborn KR (1995) MR of the brain in mitochondrial myopathy. AJNR Am J Neuroradiol 16(5):1167–1173
Barragan-Campos HM, Vallee JN, Lo D, Barrera-Ramirez CF, Argote-Greene M, Sanchez-Guerrero J, Estanol B, Guillevin R, Chiras J (2005) Brain magnetic resonance imaging findings in patients with mitochondrial cytopathies. Arch Neurol 62(5):737–742. https://doi.org/10.1001/archneur.62.5.737
Lerman-Sagie T, Leshinsky-Silver E, Watemberg N, Luckman Y, Lev D (2005) White matter involvement in mitochondrial diseases. Mol Genet Metab 84(2):127–136. https://doi.org/10.1016/j.ymgme.2004.09.008
Navarro Vázquez I, Maestre Martínez L, Lozano Setién E, Menor Serrano F (2016) Leucoencephalopathy with brain stem and spinal cord involvement and lactate elevation: report of two new cases. An Pediatr (Barc) 84(5):291–293. https://doi.org/10.1016/j.anpedi.2015.07.019
Sen A, Wattamwar PR, Thomas B, Nair M (2010) Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: a rare white matter disease with characteristic magnetic resonance imaging findings. Neurol India 58(6):958–960. https://doi.org/10.4103/0028-3886.73766
Rantamaki M, Krahe R, Paetau A, Cormand B, Mononen I, Udd B (2001) Adult-onset autosomal recessive ataxia with thalamic lesions in a Finnish family. Neurology 57(6):1043–1049. https://doi.org/10.1212/wnl.57.6.1043
Henao AI, Pira S, Herrera DA, Vargas SA, Montoya J, Castillo M (2016) Characteristic brain MRI findings in ataxia-neuropathy spectrum related to POLG mutation. Neuroradiol J 29(1):46–48. https://doi.org/10.1177/1971400915621324
Richman TR, Ermer JA, Davies SM, Perks KL, Viola HM, Shearwood AM, Hool LC, Rackham O, Filipovska A (2015) Mutation in MRPS34 compromises protein synthesis and causes mitochondrial dysfunction. PLoS Genet 11(3):e1005089. https://doi.org/10.1371/journal.pgen.1005089
Acknowledgements
We are indebted to the family members who participated in this study. We are grateful to Dr Christopher Carroll and Professor Anu Suomalainen for the information kindly provided for Western blot analysis of MRPL44.
Funding
AS is supported by a UK Medical Research Council Senior Non-Clinical Fellowship (MC_PC_13029). IJH is supported by the Ikerbasque Science Foundation, the Carlos III Health Program and the Biodonostia Research Institute. RWT is supported by the Wellcome Centre for Mitochondrial Research (203105/Z/16/Z), the Medical Research Council (MRC) International Centre for Genomic Medicine in Neuromuscular Disease, the Mitochondrial Disease Patient Cohort (UK) (G0800674), the Lily Foundation, the UK NIHR Biomedical Research Centre for Ageing and Age-related disease award to the Newcastle upon Tyne Foundation Hospitals NHS Trust, the MRC/EPSRC Molecular Pathology Node and the UK NHS Highly Specialised Service for Rare Mitochondrial Disorders of Adults and Children (http://www.newcastle-mitochondria.com/). CB is supported by the MSA Trust (JH61030/18).
Author information
Authors and Affiliations
Contributions
AH, AM, ARM, AS and HH designed and conceptualised the study. AH, AM, ARM, EB, IPH, WM, CB, ELB, LH, JMP, CEW, IDR, SS, AMP, RQ, MMR, RWT, IJH, MGH, RDSP, AS and HH performed analysis and interpreted the data. AH, AS and HH drafted the manuscript. All authors critically revised the manuscript, contributed significantly to this work and declare to meet the ICMJE criteria for authorship.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest (financial or non-financial).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horga, A., Manole, A., Mitchell, A.L. et al. Uniparental isodisomy of chromosome 2 causing MRPL44-related multisystem mitochondrial disease. Mol Biol Rep 48, 2093–2104 (2021). https://doi.org/10.1007/s11033-021-06188-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06188-1