Abstract
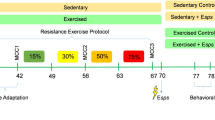
Evidence has validated the prophylactic effects of exercising on different aspects of health. On the opposite side, immobilization may lead to various destructive effects causing neurodegeneration. Here, we investigated the association between exercising and mitochondrial quality for preventing the destructive effects of restraint stress in different rat brain regions. Twenty-four male Wistar rats, were randomized into four groups (n = 6), exercise, stress, exercise + stress, and control. The exercise procedure consisted of running on a rodent treadmill for 8 weeks, and rats in the stress group were immobilized for 6 h. Rats were then euthanized by decapitation and tricarboxylic acid (TCA) cycle enzyme activity, antioxidant levels, and mitochondrial biogenesis factors were assessed in the frontal, hippocampus, parietal and temporal regions using spectrophotometer and western blot technique. Based on our results, increased activity of TCA cycle enzymes in the exercised and exercise-stressed groups was detected, except for malate dehydrogenase which was decreased in exercise-stressed group, and fumarase that did not change. Furthermore, the level of antioxidant agents (superoxide dismutase and reduced glutathione), mitochondrial biogenesis factors (peroxisome proliferator-activated receptor gamma coactivator 1-alpha and mitochondrial transcription factor A), and dynamics markers (Mitofusin 2, dynamic related protein 1, PTEN induced putative kinase-1, and parkin) increased in both mentioned groups. Interestingly our results also revealed that the majority of the mitochondrial factors increased in the frontal and parietal lobes, which may be in relation with the location of motor and sensory areas. Exercise can be used as a prophylactic approach against bioenergetics and mitochondrial dysfunction.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, FF, if required.
References
Sugama S, Sekiyama K, Kodama T et al (2016) Chronic restraint stress triggers dopaminergic and noradrenergic neurodegeneration: possible role of chronic stress in the onset of Parkinson’s disease. Brain Behav Immun 51:39–46
Sántha P, Veszelka S, Hoyk Z et al (2016) Restraint stress-induced morphological changes at the blood-brain barrier in adult rats. Front Mol Neurosci 8:88
Huang R-R, Hu W, Yin Y-Y et al (2015) Chronic restraint stress promotes learning and memory impairment due to enhanced neuronal endoplasmic reticulum stress in the frontal cortex and hippocampus in male mice. Int J Mol Med 35:553–559
Fontella FU, Vendite DA, Tabajara AS et al (2004) Repeated restraint stress alters hippocampal glutamate uptake and release in the rat. Neurochem Res 29:1703–1709
Madrigal JLM, Moro MA, Lizasoain I et al (2003) Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology 28:1579–1588
Madrigal JLM, Olivenza R, Moro MA et al (2001) Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 24:420–429
Bowman RE, Ferguson D, Luine VN (2002) effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized. Neuroscience 113:401–410
Maccari S, Morley-fletcher S (2007) Effects of prenatal restraint stress on the hypothalamus – pituitary – adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology. https://doi.org/10.1016/j.psyneuen.2007.06.005
Şahin E, Gümüşlü S (2007) Immobilization stress in rat tissues: alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comp Biochem Physiol Part C Toxicol Pharmacol 144:342–347
Bhat AH, Dar KB, Anees S et al (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110
Granata C, Jamnick NA, Bishop DJ (2018) Principles of exercise prescription, and how they influence exercise-induced changes of transcription factors and other regulators of mitochondrial biogenesis. Sport Med 1:1–19
Perry CG, Hawley JA (2017) Molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis: historical advances, current knowledge, and future challenges. Cold Spring Harb Perspect Biol 8(9):a029686
Ding Q, Vaynman S, Souda P et al (2006) Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci 24:1265–1276
Gerecke KM, Kolobova A, Allen S, Fawer JL (2013) Exercise protects against chronic restraint stress-induced oxidative stress in the cortex and hippocampus. Brain Res 1509:66–78
Naito H, Powers SK, Demirel HA, Aoki J (2001) Exercise training increases heat shock protein in skeletal muscles of old rats. Med Sci Sports Exerc 33:729–734
Khalaj L, Chavoshi Nejad S, Mohammadi M et al (2013) Assessing competence of broccoli consumption on inflammatory and antioxidant pathways in restraint-induced models: estimation in rat hippocampus and prefrontal cortex. Biomed Res Int. https://doi.org/10.1155/2013/590379
Feng P, Guan Z, Yang X, Fang J (2003) Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Res 991:195–205. https://doi.org/10.1016/j.brainres.2003.08.018
Clarke DD, Nicklas WJ, Berl S (1970) Tricarboxylic acid-cycle metabolism in brain. Effect of fluoroacetate and fluorocitrate on the labelling of glutamate, aspartate, glutamine and gamma-aminobutyrate. Biochem J 120:345–351. https://doi.org/10.1042/bj1200345
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Powell CS, Jackson RM (2003) Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am J Physiol Lung Cell Mol Physiol 285:L189–L198. https://doi.org/10.1152/ajplung.00253.2002
Vásquez-Vivar J, Kalyanaraman B, Kennedy MC (2000) Mitochondrial aconitase is a source of hydroxyl radical an electron spin resonance investigation. J Biol Chem 275:14064–14069
Gibson GE, Sheu KF, Blass JP et al (1988) Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol 45:836–840
Racker E (1950) Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. BBA Biochim Biophys Acta 4:211–214. https://doi.org/10.1016/0006-3002(50)90026-6
Wang Q, Yu L, Yu C-A (2010) Cross-talk between mitochondrial malate dehydrogenase and the cytochrome bc1 complex. J Biol Chem 285:10408–10414
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132. https://doi.org/10.1097/YCO.0b013e3280117733
Ellman GL (1959) Tissue Su ~ yd ~ l groups. Am J Anal Chem 1:70–77
Fernie AR, Carrari F, Sweetlove LJ (2004) Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 7:254–261. https://doi.org/10.1016/j.pbi.2004.03.007
Ma YS, Wu SB, Lee WY et al (2009) Response to the increase of oxidative stress and mutation of mitochondrial DNA in aging. Biochim Biophys Acta - Gen Subj 1790:1021–1029. https://doi.org/10.1016/j.bbagen.2009.04.012
Lubitz W, Ogata H (2013) Hydrogenases, structure and function. Encycl Biol Chem Second Ed. https://doi.org/10.1016/B978-0-12-378630-2.00205-X
Lee H, Yoon Y (2012) Mitochondrial dynamics: mechanisms and pathologies. eLS, American Cancer Society. https://doi.org/10.1002/9780470015902.a0021867
Liu P, Lin H, Xu Y et al (2018) Frataxin-mediated PINK1-Parkin-dependent mitophagy in hepatic steatosis: the protective effects of quercetin. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201800164
Rakovic A, Grünewald A, Kottwitz J et al (2011) Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts. PLoS ONE 6:e16746
Holloszy JO, Oscai LB, Don IJ, Mole PA (1970) Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun 40:1368–1373
Foster PP, Rosenblatt KP, Kuljiš RO (2011) Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front Neurol. https://doi.org/10.3389/fneur.2011.00028
LaNoue KF, Bryla J, Williamson JR (1972) Feedback interactions in the control of citric acid cycle activity in rat heart mitochondria. J Biol Chem 247:667–679
Minoshima S, Giordani B, Berent S et al (1997) Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc 42:85–94
Mosconi L, Tsui WH, Herholz K et al (2008) Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med Off Publ Soc Nucl Med 49:390
Sorbi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 13:72–78
Gibson GE, Shi Q (2010) A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J Alzheimer’s Dis. https://doi.org/10.3233/JAD-2010-100336
Bhattacharya SB, Datta AG (1993) Is brain a gluconeogenic organ? Mol Cell Biochem 125:51–57
Hung G-C, Brown CR, Wolfe AB et al (2004) Degradation of the gluconeogenic enzymes fructose-1, 6-bisphosphatase and malate dehydrogenase is mediated by distinct proteolytic pathways and signaling events. J Biol Chem 279:49138–49150
Yip J, Geng X, Shen J, Ding Y (2017) Cerebral gluconeogenesis and diseases. Front Pharmacol 7:521. https://doi.org/10.3389/fphar.2016.00521
Yuan X, Rietzschel N, Kwon H et al (2016) Regulation of intracellular heme trafficking revealed by subcellular reporters. Proc Natl Acad Sci U S A 113:E5144–E5152. https://doi.org/10.1073/pnas.1609865113
Benarroch EE (2009) Brain iron homeostasis and neurodegenerative disease. Neurology 72:1436–1440
Freitas HR, Ferraz G, Ferreira GC et al (2016) Glutathione-induced calcium shifts in chick retinal glial cells. PLoS ONE 11:1–20. https://doi.org/10.1371/journal.pone.0153677
Bugg JM, Head D (2011) Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging 32:506–514
Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25:1354–1366
Kim JH, Shin HD, Park BL et al (2005) Peroxisome proliferator-activated receptor gamma coactivator 1 alpha promoter polymorphisms are associated with early-onset type 2 diabetes mellitus in the Korean population. Diabetologia 48:1323–1330. https://doi.org/10.1007/s00125-005-1793-4
Robinson MM, Lowe VJ, Nair KS (2018) Increased brain glucose uptake after 12 weeks of aerobic high-intensity interval training in young and older adults. J Clin Endocrinol Metab 103:221–227. https://doi.org/10.1210/jc.2017-01571
Twig G, Shirihai OS (2010) The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14:1939–1951. https://doi.org/10.1089/ars.2010.3779
Acknowledgements
This work was supported by The Research grant of Shahid Beheshti University of Medical Sciences (No.11844‐2).
Funding
This work was supported by The Research grant of Shahid Beheshti University of Medical Sciences (No.11844‐2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The experiments were performed after approval by the Ethics Committee of the institution (Ethics code: IR.SBMU.PHNS.REC.1396.34).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khodagholi, F., Zareh Shahamati, S., Maleki Chamgordani, M. et al. Interval aerobic training improves bioenergetics state and mitochondrial dynamics of different brain regions in restraint stressed rats. Mol Biol Rep 48, 2071–2082 (2021). https://doi.org/10.1007/s11033-021-06177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06177-4