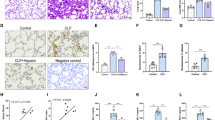
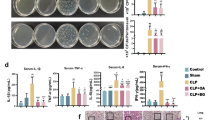
Abstract
Cardiac fibroblasts (CFs) have a key role in the inflammatory response after cardiac injury and are necessary for wound healing. Resolvins are potent agonists that control the duration and magnitude of inflammation. They decrease mediators of pro-inflammatory expression, reduce neutrophil migration to inflammation sites, promote the removal of microbes and apoptotic cells, and reduce exudate. However, whether resolvins can prevent pro-inflammatory-dependent effects in CFs is unknown. Thus, the present work was addressed to study whether resolvin D1 and E1 (RvD1 and RvE1) can prevent pro-inflammatory effects on CFs after lipopolysaccharide (LPS) challenge. For this, CFs were stimulated with LPS, in the presence or absence of RvD1 or RvE1, to analyze its effects on intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion protein 1 (VCAM-1), monocyte adhesion and the cytokine levels of tumor necrosis factor alpha (TNF-α), interleukin-6(IL-6), interleukin-1beta (IL-1β), monocyte chemoattractant protein-1 (MCP-1) and interleukin-10 (IL-10). Our results showed that CFs are expressing ALX/FPR2 and ChemR23, RvD1 and RvE1 receptors, respectively. RvD1 and RvE1 prevent the increase of ICAM-1 and VCAM-1 protein levels and the adhesion of spleen mononuclear cells to CFs induced by LPS. Finally, RvD1, but not RvE1, prevents the LPS-induced increase of IL-6, MCP-1, TNF-α, and IL-10. In conclusion, our findings provide evidence that in CFs, RvD1 and RvE1 might actively participate in the prevention of inflammatory response triggered by LPS.




Similar content being viewed by others
References
Frangogiannis N (2006) The mechanistic basis of infarct healing. Antiox Redox Sign 8(11–12):1907–1939. https://doi.org/10.1089/ars.2006.8.1907
Díaz-Araya G, Vivar R, Humeres C et al (2015) Cardiac fibroblasts as sentinel cells in cardiac tissue: receptors, signaling pathways and cellular functions. Pharmacol Res 101:30–40. https://doi.org/10.1016/j.phrs.2015.07.001
Yang R, Song Z, Wu S, Wei Z, Xu Y, Shen X (2018) Toll-like receptor 4 contributes to a myofibroblast phenotype in cardiac fibroblasts and is associated with autophagy after myocardial infarction in a mouse model. Atherosclerosis. 279:23–31. https://doi.org/10.1016/j.atherosclerosis.2018.10.018
Humeres C, Vivar R, Boza P et al (2016) Cardiac fibroblast cytokine profiles induced by proinflammatory or profibrotic stimuli promote monocyte recruitment and modulate macrophage M1/M2 balance in vitro. J Mol Cell Cardiol 101:69–80. https://doi.org/10.1016/j.yjmcc.2016.10.014
Dewald O, Zymek P, Winkelmann K et al (2005) CCL2/Monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96:881–889. https://doi.org/10.1161/01.RES.0000163017.13772.3a
Nahrendorf M, Swirski F, Aikawa E et al (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204(12):3037–3047. https://doi.org/10.1084/jem.20070885
Serhan CN, Brain SD, Buckley CD et al (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J 21(2):325–332. https://doi.org/10.1096/fj.06-7227rev
Buckley CD, Gilroy DW, Serhan CN (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 40(3):315–327. https://doi.org/10.1016/j.immuni.2014.02.009
Calder P (2017) Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 45(5):1105–1115. https://doi.org/10.1042/BST20160474
Serhan C, Krishnamoorthy S, Recchiuti A, Chiang N (2011) Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr Top Med Chem 11(6):629–647. https://doi.org/10.2174/1568026611109060629
Serhan CN, Levy BD (2018) Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 128(7):2657–2669. https://doi.org/10.1172/JCI97943
Liu G, Liu Q, Shen Y, Kong D, Gong Y, Tao B, Chen G, Guo S, Li J, Zuo S, Yu Y, Yin H, Zhang L, Zhou B, Funk CD, Zhang J, Yu Y (2018) Early treatment with resolvin E1 facilitates myocardial recovery from ischaemia in mice. Br J Pharmacol 175(8):1205–1216. https://doi.org/10.1111/bph.14041
Salic K, Morrison M, Verschuren L, Wielinga P, Wu L, Kleemann R, Gjorstrup P, Kooistra T (2016) Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 250:158e165
Fredman G, Van Dyke T, Serhan C (2010) Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol 30:2005e2013
Keyes K, Ye Y, Lin Y et al (2010) Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circul Physiol 299(1):H153–H164. https://doi.org/10.1152/ajpheart.01057.2009
Wu B, Mottola G, Chatterjee A, Lance K, Chen M, Siguenza I, Desai T, Conte M (2017) Perivascular delivery of resolvin D1 inhibits neointimal hyper- plasia in a rat model of arterial injury. J Vasc Surg 65(1):207-217.e3
Gilbert K, Bernier J, Godbout R, Rousseau G (2014) Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar Drugs 12:5396e5407
Kain V, Halade GV (2019) Immune responsive resolvin D1 programs peritoneal macrophages and cardiac fibroblast phenotypes in diversified metabolic microenvironment. J Cell Physiol 234(4):3910–3920. https://doi.org/10.1002/jcp.27165
Olivares-Silva F, Landaeta R, Aránguiz P et al (2018) Heparan sulfate potentiates leukocyte adhesion on cardiac fibroblast by enhancing VCAM-1 and ICAM-1 expression. Biochim Biophys Acta Mol basis Dis 1864(3):831–842. https://doi.org/10.1016/j.bbadis.2017.12.002
Vivar R, Humeres C, Varela M et al (2012) Cardiac fibroblast death by ischemia/reperfusion is partially inhibited by IGF-1 through both PI3K/Akt and MEK–ERK pathways. Exp Mol Pathol 93:1–7. https://doi.org/10.1016/j.yexmp.2012.01.010
Karatay E, Utku ÖG (2020) Serum resolvin D1 levels as a marker of inflammation in constipation dominant irritable bowel syndrome. Turk J Gastroenterol 31(2):113–119. https://doi.org/10.5152/tjg.2020.19751
Bolívar S, Santana R, Ayala P, Landaeta R, Boza P, Humeres C, Vivar R, Muñoz C, Pardo V, Fernandez S, Anfossi R, Diaz-Araya G (2017) Lipopolysaccharide activates toll-like receptor 4 and prevents cardiac fibroblast-to-myofibroblast differentiation. Cardiovasc Toxicol 17(4):458–470. https://doi.org/10.1007/s12012-017-9404-4
Chen J, Shetty S, Zhang P et al (2014) Aspirin-triggered resolvin D1 down-regulates inflammatory responses and protects against endotoxin-induced acute kidney injury. Toxicol Appl Pharmacol 277(2):118–123. https://doi.org/10.1016/j.taap.2014.03.017
Tian H, Lu Y, Sherwood A et al (2009) Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci 50(8):3613–3620. https://doi.org/10.1167/iovs.08-3146
Miyahara T, Runge S, Chatterjee A et al (2013) D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J 27(6):2220–2232. https://doi.org/10.1096/fj.12-225615
Cox R, Phillips O, Fukumoto J et al (2015) Resolvins decrease oxidative stress mediated macrophage and epithelial cell interaction through decreased cytokine secretion. PLoS One 10(8):e0136755. https://doi.org/10.1371/journal.pone.0136755
Segal A (2005) How neutrophils kill microbes. Annu Rev Immunol 23:197–223. https://doi.org/10.1146/annurev.immunol.23.021704.115653
Zhang Z, Hu X, Qi X et al (2018) Resolvin D1 promotes corneal epithelial wound healing and restoration of mechanical sensation in diabetic mice. Mol Vis 24:274–285
Hsiao H, Sapinoro R, Thatcher T et al (2013) A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One 8(3):e58258. https://doi.org/10.1371/journal.pone.0058258
Gu Z, Lamont G, Lamont R et al (2016) Resolvin D1, resolvin D2 and maresin 1 activate the GSK3β anti-inflammatory axis in TLR4-engaged human monocytes. Inn Immunol 22(3):186–195. https://doi.org/10.1177/1753425916628618
Khaled M, Alshibani N, Labban N et al (2013) Effects of resolvin D1 on cell survival and cytokine expression of human gingival fibroblasts. Abstract J Periodontol 84(12):1838–1846. https://doi.org/10.1902/jop.2013.120388
Li G, Chen Z, Bhat O et al (2017) NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J Lipid Res 58(6):1080–1090. https://doi.org/10.1194/jlr.M072587
Arita M, Ohira T, Sun Y et al (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 178(6):3912–3917. https://doi.org/10.4049/jimmunol.178.6.3912
Xu H, Chen J, Ge J et al (2019) Resolvin E1 ameliorates pulpitis by suppressing dental pulp fibroblast activation in a chemerin receptor 23-dependent manner. J Endod 45(9):1126-1134.e1. https://doi.org/10.1016/j.joen.2019.05.005
Lund T, Mangsbo S, Scholz H et al (2010) Resolvin E1 reduces proinflammatory markers in human pancreatic islets in vitro. Exp Clin Endocrinol Diab 118:237–244. https://doi.org/10.1055/s-0029-1241825
Baker L, Martin N, Kimber M et al (2018) Rv E1 attenuates LPS induced inflammation and subsequent atrophy in C2C12 myotubes. J Cell Biochem 119(7):6094–6103
Levy B (2012) Resolvin D1 and resolvin E1 promote the resolution of allergic airway inflammation via shared and distinct molecular counter-regulatory pathways. Front Immunol 3:390. https://doi.org/10.3389/fimmu.2012.00390
Kaye R, Botten N, Lippestad M et al (2019) Resolvin D1, but not resolvin E1, transactivates the epidermal growth factor receptor to increase intracellular calcium and glycoconjugate secretion in rat and human conjunctival goblet cells. Exp Eye Res 180:53–62. https://doi.org/10.1016/j.exer.2018.11.018
Kasuga K, Yang R, Porter TF et al (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol 181(12):8677–8687. https://doi.org/10.4049/jimmunol.181.12.8677
Rey C, Nadjar A, Buaud B, Vaysse C, Aubert A, Pallet V et al (2016) Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun 55:249–259. https://doi.org/10.1016/j.bbi.2015.12.013
Mou H, Li Z, Kong Y et al (2011) Proinflammatory stimulants promote the expression of a promiscuous G protein-coupled receptor, mFPR2, in microvascular endothelial cells. Inflammation 35(2):656–664. https://doi.org/10.1007/s10753-011-9358-9
Cui Y, Le Y, Zhang X et al (2002) Up-regulation of FPR2, a chemotactic receptor for amyloid β 1–42 (Aβ42), in murine microglial cells by TNFα. Neurobiol Dis 10(3):366–377. https://doi.org/10.1006/nbdi.2002.0517
Zhu M, Wang X, Schultzberg M, Hjorth E (2015) Differential regulation of resolution in inflammation induced by amyloid-β42 and lipopolysaccharides in human microglia. J Alz Dis 43(4):1237–1250. https://doi.org/10.3233/jad-141233
Freire M, Dalli J, Serhan C, VanDyke T (2017) Neutrophil resolvin E1 receptor expression and function in type 2 diabetes. J Immunol 198(2):718–728. https://doi.org/10.4049/jimmunol.1601543
Herová M, Schmid M, Gemperle C, Hersberger M (2015) ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol 194(5):2330–2337. https://doi.org/10.4049/jimmunol.1402166
Cao D, Pi J, Shan Y et al (2018) Anti-inflammatory effect of resolvin D1 on LPS-treated MG-63 cells. Exp Ther Med 16(5):4283–4288. https://doi.org/10.3892/etm.2018.6721
Raabe C, Gröper J, Rescher U (2019) Biased perspectives on formyl peptide receptors. Biochimica et Biophysica Acta (BBA) Mol Cell Res 1866(2):305–316. https://doi.org/10.1016/j.bbamcr.2018.11.015
Laranjeira S, Regan-Komito D, Iqbal A et al (2017) A model for the optimization of anti-inflammatory treatment with chemerin. Interf Focus 8(1):1–12. https://doi.org/10.1098/rsfs.2017.0007
Wang Y, Huo J, Zhang D et al (2019) Chemerin/ChemR23 axis triggers an inflammatory response in keratinocytes through ROS-sirt1-NF-κB signaling. J Cell Biochem 120:6459–6470. https://doi.org/10.1002/jcb.27936
Demarquoy J, Le Borgne F (2014) Biosynthesis, metabolism and function of protectins and resolvins. Clin Lipidol 9(6):683–693 Patil VS, Magar NG (1960) Fatty acids of human blood. Biochem J 74:427–429
Funding
This work was supported by FONDECYT grant 1170425 from “Agencia Nacional de Investigación y Desarrollo de Chile (ANID)”, and fellowships OAICE-CAB-03-031-2015 from “Universidad de Costa Rica” and PED-118-2015-I from “Ministerio de Ciencia, Tecnología y Telecomunicaciones de Costa Rica”.
Author information
Authors and Affiliations
Contributions
Aimée Salas-Hernández: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing—Original Draft, Writing—Review & Editing. Claudio Espinoza: Methodology, Investigation, Formal analysis. Raúl Vivar: Methodology. Jenaro Espitia-Corredor: Methodology. José Lillo: Methodology. Pablo Parra-Flores: Methodology. Carlos Sánchez Ferrer: Conceptualization, Methodology, Writing—Review & Editing Resources. Cocepción Peiró: Conceptualization, Methodology, Investigation, Writing—Review & Editing. Diaz-Araya G: Conceptualization, Methodology, Investigation, Writing—Review & Editing, Supervision Project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Experimental protocols following international Guiding Principles for Biomedical Research Involving Animals were approved by the Bioethics Committee for Animal Research of the Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile (CBE2017-08).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salas-Hernández, A., Espinoza-Pérez, C., Vivar, R. et al. Resolvin D1 and E1 promote resolution of inflammation in rat cardiac fibroblast in vitro. Mol Biol Rep 48, 57–66 (2021). https://doi.org/10.1007/s11033-020-06133-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06133-8