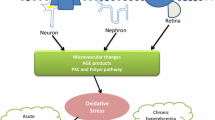
Abstract
Diabetes mellitus (DM) has become one of the major healthcare challenges worldwide in the recent times and inflammation being one of its key pathogenic process/mechanism affect several body parts including the peripheral and central nervous system. High-mobility group box 1 (HMGB1) is one of the major non-histone proteins that plays a key role in triggering the inflammatory response. Upon its release into the extracellular milieu, HMGB1 acts as an “alarmin” for the immune system to initiate tissue repair as a component of the host defense system. Furthermore, HMGB1 along with its downstream receptors like Toll-like receptors (TLRs) and receptors for advanced glycation end products (RAGE) serve as the suitable target for DM. The forthcoming research in the field of diabetes would potentially focus on the development of alternative approaches to target the centre of inflammation that is primarily mediated by HMGB1 to improve diabetic-related complications. This review covers the therapeutic actions of HMGB1 protein, which acts by activating the RAGE and TLR molecules to constitute a functional tripod system, in turn activating NF-κB pathway that contributes to the production of mediators for pro-inflammatory cytokines associated with DM. The interaction between TLR2 and TLR4 with ligands present in the host and the activation of RAGE stimulates various immune and metabolic responses that contribute to diabetes. This review emphasizes to elucidate the role of HMGB1 in the initiation and progression of DM and control over the inflammatory tripod as a promising therapeutic approach in the management of DM.
Graphic abstract




Similar content being viewed by others
References
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14:88–98. https://doi.org/10.1038/nrendo.2017.151
Wang Y, Zhong J, Zhang X et al (2016) The role of HMGB1 in the pathogenesis of type 2 diabetes. J Diabetes Res 2016:2543268. https://doi.org/10.1155/2016/2543268
Liu C, Feng X, Li Q, Wang Y et al (2016) Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine 86:100–109. https://doi.org/10.1016/j.cyto.2016.06.028
Wang X, Bao W, Liu J et al (2013) Inflammatory markers and risk of type 2 diabetes. Diabetes Care 36:166–175. https://doi.org/10.2337/dc12-0702
Massaro M, Scoditti E, Pellegrino M et al (2016) Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)α/γ agonist aleglitazarin attenuating TNF-α-mediated inflammation and insulin resistance in human adipocytes. Pharmacol Res 107:125–136. https://doi.org/10.1016/j.phrs.2016.02.027
Haraba R, Suica VI, Uyy E et al (2011) Antohe: Hyperlipidemia stimulates the extracellular release of the nuclear high mobility group box 1 protein. Cell Tissue Res 346:361–368. https://doi.org/10.1007/s00441-011-1277-4
Biscetti F, Ghirlanda G, Flex A (2011) Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration. Curr Vasc Pharmacol 9:677–681. https://doi.org/10.2174/157016111797484125
Tsung A, Tohme S, Billiar TR (2014) High-mobility group box-1 in sterile inflammation. J Intern Med 276:425–443. https://doi.org/10.2174/157016111797484125
Musumeci D, Roviello GN, Montesarchio D (2014) An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther 141:347–357. https://doi.org/10.1016/j.pharmthera.2013.11.001
Malarkey CS, Churchill M (2012) The high mobility group box: the ultimate utility player of a cell. Trends Biochem Sci 37:553–562. https://doi.org/10.1016/j.tibs.2012.09.003
Yang L, Xie M, Yang M et al (2014) PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 5:4436. https://doi.org/10.1038/ncomms5436
Kang R, Zhang Q, Zeh HJ, Lotze MT, Tang D (2013) HMGB1 in cancer: good, bad, or both? Clin Cancer Res 19:4046–4057. https://doi.org/10.1158/1078-0432.CCR-13-0495
Vijayakumar EC, Bhatt LK, Prabhavalkar KS (2019) High Mobility Group Box-1 (HMGB1): a potential target in therapeutics. Curr Drug Targets 20:1474–1485. https://doi.org/10.2174/1389450120666190618125100
Wu H, Chen Z, Xie J et al (2016) High Mobility Group Box-1: a missing link between diabetes and its complications. Mediat Inflamm 2016:3896147. https://doi.org/10.1155/2016/3896147
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395:203–230. https://doi.org/10.1515/hsz-2013-0241
Wang H, Qu H, Deng H (2015) Plasma HMGB-1 levels in subjects with obesity and type 2 diabetes: a cross-sectional study in China. PLoS One 10:e0136564. https://doi.org/10.1371/journal.pone.0136564
Dasu MR, Devaraj S, Park S, Jialal I (2010) Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33:861–868. https://doi.org/10.2337/dc09-1799
Dandona P, Ghanim H, Green K, Sia CL et al (2013) Insulin infusion suppresses while glucose infusion induces toll-like receptors and high-mobility group-B1 protein expression in mononuclear cells of type 1 diabetes patients. Am J Physiol Endocrinol Metab 304:E810–E818. https://doi.org/10.1152/ajpendo.00566.2012
Gay NJ, Symmons MF, Gangloff M, Bryant CE (2014) Assembly and localization of toll-like receptor signalling complexes. Nat Rev Immunol 14:546. https://doi.org/10.1038/nri3713
Zhang S, Zhong J, Yang P, Gong F, Wang CY (2010) HMGB1, an innate alarmin, in the pathogenesis of type 1 diabetes. Int J Clin Exp Pathol 3:24–38. https://doi.org/10.7326/M20-0533
Wu H, Li R, Wei ZH, Zhang XL et al (2016) Diabetes-induced oxidative stress in endothelial progenitor cells may be sustained by a positive feedback loop involving high mobility group box-1. Oxidative Med Cell Longev 2016:1943918. https://doi.org/10.1155/2016/1943918
Yang D, Tewary P, Rosa GDL, Wei F, Oppenheim JJ (2010) The alarmin functions of high-mobility group proteins. Biochim Biophys Acta 1799(1–2):157–163. https://doi.org/10.1016/j.bbagrm.2009.11.002
Tang D, Billiar TR, Lotze MT (2012) A Janus tale of two active High Mobility Group Box 1 (HMGB1) redox states. Mol Med 18:1360–1362. https://doi.org/10.2119/molmed.2012.00314
Venereau E, Casalgrandi M, Schiraldi M et al (2012) Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 209:1519–1528. https://doi.org/10.1084/jem.20120189
Ibrahim ZA, Armour CL, Phipps S, Sukkar MB (2013) RAGE and TLRs: relatives, friends or neighbours? Mol Immunol 56:739–744. https://doi.org/10.1016/j.molimm.2013.07.008
Giovannini S, Tinelli G, Biscetti F et al (2017) Serum high mobility group box-1 and osteoprotegerin levels are associated with peripheral arterial disease and critical limb ischemia in type 2 diabetic subjects. Cardiovasc Diabetol 16:99. https://doi.org/10.1186/s12933-017-0581-z
Yang S-L, Zhu L-Y, Han R et al (2017) Pathophysiology of peripheral arterial disease in diabetes mellitus. J Diabetes 9:133–140. https://doi.org/10.1111/1753-0407.12474
Nadeau-Vallée M, Obari D, Palacios J et al (2016) Sterile inflammation and pregnancy complications: a review. Reproduction 152:277–292. https://doi.org/10.1530/REP-16-0453
Venereau E, Schiraldi M, Uguccioni M, Bianchi ME (2013) HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol 55:76–82. https://doi.org/10.1016/j.molimm.2012.10.037
Magna M, Pisetsky DS (2014) The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med 20:138–146. https://doi.org/10.2119/molmed.2013.00164
Yanai H, Matsuda A, Koshiba R, Nishio J et al (2013) Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. PNAS 110:20699–20704. https://doi.org/10.1073/pnas.1320808110
Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME (2014) A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med 20:135–137. https://doi.org/10.2119/molmed.2014.00022
Schiraldi M, Raucci A, Munoz LM et al (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209:551–563. https://doi.org/10.1084/jem.20111739
Di Maggio S, Milan G, De Marchis F et al (2017) Non-oxidizable HMGB1 induces cardiac fibroblasts migration via CXCR4 in a CXCL12-independent manner and worsens tissue remodeling after myocardial infarction. Biochim Biophys Acta 1863:2693–2704. https://doi.org/10.1016/j.bbadis.2017.07.012
Vogel S, Rath D, Borst O et al (2016) Platelet-derived highmobility group box 1 promotes recruitment and suppresses apoptosis of monocytes. Biochem Biophys Res Commun 478:143–148. https://doi.org/10.1016/j.bbrc.2016.07.078
Yang H, Antoine DJ, Andersson U, Tracey KJ (2013) The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93:865–873. https://doi.org/10.1016/j.bbrc.2016.07.078
Ma F, Kouzoukas DE, Meyer-Siegler KL et al (2017) Disulfide high mobility group box-1 causes bladder pain through bladder Toll-like receptor 4. BMC Physiol 17:6. https://doi.org/10.1186/s12899-017-0032-9
Kianian F, Kadkhodaee M, Sadeghipour HR, Karimian SM et al (2020) An overview of high-mobility group box 1, a potent pro-inflammatory cytokine in asthma. J Basic Clin Physiol Pharmacol 31(6):20190363. https://doi.org/10.1515/jbcpp-2019-0363
Frank MG, Weber MD, Fonken LK et al (2016) The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: a role for the NLRP3 inflammasome. Brain Behav Immun 55:215–224. https://doi.org/10.1016/j.bbi.2015.10.009
Stark K, Philippi V, Stockhausen S et al (2016) Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 128:2435–2449. https://doi.org/10.1182/blood-2016-04-710632
Yang H, Lundback P, Ottosson L et al (2012) Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 18:250–259. https://doi.org/10.2119/molmed.2011.00389
Singh B, Biswas I, Bhagat S, Surya Kumari S, Khan GA (2016) HMGB1 facilitates hypoxia-induced WF upregulation through TLR2MYD88-SP1 pathway. Eur J Immunol 46:2388–2400. https://doi.org/10.1002/eji.201646386
Yao H, Hu C, Yin L et al (2016) Dioscin reduces lipopolysaccharide-induced inflammatory liver injury via regulating TLR4/MyD88 signal pathway. Int Immunopharmacol 36:132–141. https://doi.org/10.1016/j.intimp.2016.04.023
Arrigo T, Chirico V, Salpietro V et al (2013) High-mobility group protein B1: a new biomarker of metabolic syndrome in obese children. Eur J Endocrinol 168(4):631–638
Montes VN, Subramanian S, Goodspeed L et al (2015) Anti-HMGB1 antibody reduces weight gain in mice fed a high-fat diet. Nutr Diabetes 5:e161
Sumiyoshi M, Satomi J, Kitazato KT, Yagi K et al (2015) PPARγ dependent and -independent inhibition of the HMGB1/TLR9 pathway by eicosapentaenoic acid attenuates ischemic brain damage in ovariectomized rats. J Stroke Cerebrovasc Dis 24:1187–1195. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.01.009
Song F, Del Pozo CH, Rosario R et al (2014) RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes 63(6):1948–1965
Xie HL, Zhang Y, Huang YZ, Li S et al (2014) Regulation of high mobility group box 1 and hypoxia in the migration of mesenchymal stem cells. Cell Biol Int 38:892–897. https://doi.org/10.1002/cbin.10279
Yu M, Wang H, Ding A et al (2006) HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26:174–179. https://doi.org/10.1189/jlb.0306180
Kang R, Livesey KM, Zeh HJ, Loze MT, Tang D (2010) HMGB1: a novel beclin 1-binding protein active in autophagy. Autophagy 6:1209–1211. https://doi.org/10.4161/auto.6.8.13651
Kim S, Kim SY, Pribis JP et al (2013) Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med 19:88–98. https://doi.org/10.2119/molmed.2012.00306
Yamashita A, Nishihira K, Matsuura Y et al (2012) Paucity of CD34-positive cells and increased expression of high-mobility group box 1 in coronary thrombus with type 2 diabetes mellitus. Atherosclerosis 224:511–514. https://doi.org/10.1016/j.atherosclerosis.2012.07.027
Syed MA, Barinas-Mitchell E, Pietropaolo SL et al (2002) Is type 2 diabetes a chronic inflammatory/autoimmune disease? Diabetes Nutr Metab 15:68–83. https://doi.org/10.1242/dmm.036004
Matsuoka N, Itoh T, Watari H et al (2010) High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest 120:735–743. https://doi.org/10.1172/JCI41360
Yang H, Hreggvidsdottir HS, Palmblad K et al (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942–11947. https://doi.org/10.1073/pnas.1003893107
Yamamoto M, Takeda K (2010) Current views of toll-like receptor signaling pathways. Gastroenterol Res Pract 2010:240365. https://doi.org/10.1155/2010/240365
Ruan BH, Li X, Winkler AR et al (2010) Complement C3a, CpG oligos, and DNA/C3a complex stimulate IFN-alpha production in a receptor for advanced glycation end product-dependent manner. J Immunol 185:4213–4222. https://doi.org/10.4049/jimmunol.1000863
Yamamoto Y, Harashima A, Saito H et al (2011) Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J Immunol 186:3248–3257. https://doi.org/10.4049/jimmunol.1002253
Bierhaus A, Nawroth PP (2009) Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 52:2251–2263. https://doi.org/10.1007/s00125-009-1458-9
Wang CY, Podolsky R, She JX (2006) Genetic and functional evidence supporting SUMO4 as a type 1 diabetes susceptibility gene. Ann N Y Acad Sci 1079:257–267. https://doi.org/10.1196/annals.1375.039
Yang H, Wang H, Czura CJ, Tracey KJ (2005) The cytokine activity of HMGB1. J Leukoc Biol 78(1):1–8
Pietropaolo M, Barinas-Mitchell E, Kuller LH (2007) The heterogeneity of diabetes: unraveling a dispute: is systemic inflammation related to islet autoimmunity? Diabetes 56:1189–1197. https://doi.org/10.2337/db06-0880
Kazama H, Ricci JE, Herndon JM et al (2008) Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of highmobility group box-1 protein. Immunity 29:21–32. https://doi.org/10.1016/j.immuni.2008.05.013
Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P (2007) The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 81:84–91. https://doi.org/10.1189/jlb.0306171
Telusma G, Datta S, Mihajlov I et al (2006) Dendritic cell activating peptides induce distinct cytokine profiles. Int Immunol 18:1563–1573
Yang D, Chen Q, Yang H et al (2007) High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 81:59–66. https://doi.org/10.1189/jlb.0306180
Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D et al (2006) High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 290:C917–C924. https://doi.org/10.1152/ajpcell.00401.2005
Kawai T, Akira S (2007) Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13:460–469. https://doi.org/10.1016/j.molmed.2007.09.002
Ivanov S, Dragoi AM, Wang X et al (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110:1970–1981. https://doi.org/10.1182/blood-2006-09-044776
Han J, Zhong J, Wei W, Wang Y et al (2008) Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes 57:2118–2127. https://doi.org/10.2337/db07-1499
O’Brien BA, Geng X, Orteu GH et al (2006) A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun 26:104–115. https://doi.org/10.1016/j.jaut.2005.11.006
Moser B, Szabolcs MJ, Ankersmit HJ et al (2007) Blockade of RAGE suppresses alloimmune reactions in vitro and delays allograft rejection in murine heart transplantation. Am J Transplant 7:293–302. https://doi.org/10.1111/j.1600-6143.2006.01617.x
Yi S, Wang Y, Chandra AP et al (2007) Requirement of MyD88 for macrophage-mediated islet xenograft rejection after adoptive transfer. Transplantation 83:615–623. https://doi.org/10.1097/01.tp.0000253759.87886.39
Goldberg A, Parolini M, Chin BY et al (2007) Toll-like receptor 4 suppression leads to islet allograft survival. FASEB J 21:2840–2848. https://doi.org/10.1096/fj.06-7910com
Chen Y, Akirav EM, Chen W et al (2008) RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol 181:4272–4278. https://doi.org/10.4049/jimmunol.181.6.4272
Inoue K, Kawahara K, Biswas KK et al (2007) Hmgb1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol 1(6):136–143. https://doi.org/10.1016/j.carpath.2006.11.006
Penfold SA, Coughlan MT, Patel SK et al (2010) Circulating high-molecular-weight rage ligands activate pathways implicated in the development of diabetic nephropathy. Kidney Int 7(8):287–295. https://doi.org/10.1038/ki.2010.134
Sohn EJ, Kim CS, Kim YS et al (2007) Effects of magnolol (5,5 diallyl-2,2′-dihydroxybiphenyl) on diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. Life Sci 80:468–475. https://doi.org/10.1016/j.lfs.2006.09.037
Abdelsadik A, Trad A (2011) Toll-like receptors on the fork roads between innate and adaptive immunity. Hum Immunol 72:1188–1193. https://doi.org/10.1016/j.humimm.2011.08.015
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846
Clynes R, Moser B, Yan SF et al (2007) Receptor for age (RAGE): weaving tangled webs within the inflammatory response. Curr Mol Med 7:743–751. https://doi.org/10.2174/156652407783220714
Thornalley PJ (2007) Dietary ages and ales and risk to human health by their interaction with the receptor for advanced glycation end products (RAGE) – an introduction. Mol Nutr Food Res 5(1):1107–1110. https://doi.org/10.1002/mnfr.200700017
Ramasamy R, Yan SF, Schmidt AM (2007) Arguing for the motion: yes, RAGE is a receptor for advanced glycation end products. Mol Nutr Food Res 5(1):1111–1115. https://doi.org/10.1002/mnfr.200700008
Heizmann CW (2007) The mechanism by which dietary ages are a risk to human health is via their interaction with rage: arguing against the motion. Mol Nutr Food Res 5(1):1116–1119. https://doi.org/10.1002/mnfr.200600284
Foell D, Wittkowski H, Vogl T, Roth J (2007) S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 8(1):28–37. https://doi.org/10.1189/jlb.0306170
Yuan M, Konstantopoulos N, Lee J et al (2001) Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293(5535):1673–1677
Zheng L, Sinniah R, Hsu SI (2008) Pathogenic role of NF-kappaB activation in tubulointerstitial inflammatory lesions in human lupus nephritis. J Histochem Cytochem 5(6):517–529 10.1369%2Fjhc.7A7368.2008
Göser S, Andrassy M, Buss SJ et al (2006) Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation 114(16):1693–1702. https://doi.org/10.1161/CIRCULATIONAHA.106.635664
Chen Y, Qiao F, Zhao Y, Wang Y, Liu G (2015) HMGB1 is activated in type 2 diabetes mellitus patients and in mesangial cells in response to high glucose. Int J Clin Exp Pathol 8(6):6683–6691
Böni-Schnetzler M, Boller S, Debray S et al (2009) Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150(12):5218–5229
Dumitriu IE, Baruah P, Valentinis B et al (2005) Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174(12):7506–7515. https://doi.org/10.4049/jimmunol.174.12.7506
Jaulmes A, Thierry S, Janvier B, Raymondjean M (2006) Activation of sPLA2-IIA and PGE2 production by high mobility group protein B1 in vascular smooth muscle cells sensitized by IL-1beta. FASEB J 20(10):1727–1729. https://doi.org/10.1096/fj.05-5514fje
Mitola S, Belleri M, Urbinati C et al (2006) Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol 176(1):12–15. https://doi.org/10.4049/jimmunol.176.1.12
Harja E, Bu DX, Hudson BI et al (2008) Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest 118(1):183–194. https://doi.org/10.1172/JCI32703
Talchai C, Xuan S, Lin HV, Sussel L, Accili D (2012) Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150(6):1223–1234
Hudson BI, Kalea AZ, Del Mar A et al (2008) Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem 283:34457–34468. https://doi.org/10.1016/j.cclet.2018.04.005
Ramasamy R, Yan SF, Schmidt AM (2009) RAGE: therapeutic target and biomarker of the inflammatory response-the evidence mounts. J Leukoc Biol 86:505–512. https://doi.org/10.1189/jlb.0409230
Nativel B et al (2013) Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: contribution to chronic inflammation in fat tissue. PLoS One 8(9):e76039. https://doi.org/10.1371/journal.pone.0076039
Soro-Paavonen A, Watson AMD, Li J et al (2008) Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 57:2461–2469. https://doi.org/10.2337/db07-1808
Volz HC, Seidel C, Laohachewin D, Kaya Z et al (2010) HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol 105:805–820. https://doi.org/10.1007/s00395-010-0114-3
Wang WK, Lu QH, Zhang JN et al (2014) HMGB1mediates hyperglycaemia-induced cardiomyocyte apoptosis via ERK/Ets-1signalling pathway. J Cell Mol Med 18:2311–2320. https://doi.org/10.1111/jcmm.12399
Tao A, Song J, Lan T, Xu X et al (2015) Cardiomyocyte-fibroblast interaction contributes to diabetic cardiomyopathy in mice: Role of HMGB1/TLR4/IL-33 axis. Biochim Biophys Acta 1852:2075–2085. https://doi.org/10.1016/j.bbadis.2015.07.015
Jiang S, Chen X (2017) HMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivo. Drug Des Devel Ther 11:783–795. https://doi.org/10.2147/DDDT.S129913
Zhang W, Wang Y, Kong Y (2019) Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Investig Ophthalmol Vis Sci 60:294–303. https://doi.org/10.1167/iovs.18-25617
Liu L, Patel P, Steinle JJ (2018) PKA regulates HMGB1 through activation of IGFBP-3 and SIRT1 in human retinal endothelial cells cultured in high glucose. Inflamm Res Off J Eur Histamine Res Soc 67:1013–1019. https://doi.org/10.1007/s00011-018-1196-x
Raucci A, Di Maggio S, Scavello F et al (2019) The Janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci 76:211–229. https://doi.org/10.1007/s00018-018-2930-9
Wang W-K, Wang B, Lu Q-H, Zhang W et al (2014) Inhibition of high-mobility group box 1 improves myocardial fibrosis and dysfunction in diabetic cardiomyopathy. Int J Cardiol 172:202–212. https://doi.org/10.1016/j.ijcard.2014.01.011
Heng LZ, Comyn O, Peto T et al (2013) Diabetic retinopathy: Pathogenesis, clinical grading, management and future developments. Diabet Med J Br Diabet Assoc 30:640–650. https://doi.org/10.1111/dme.12089
Hu J, Liu B, Zhao Q, Jin P et al (2016) Bone marrow stromal cells inhibits HMGB1-mediated inflammation after stroke in type 2 diabetic rats. Neuroscience 324:11–19. https://doi.org/10.1016/j.neuroscience.2016.02.058
Coughlan MT, Thorburn DR, Penfold SA et al (2009) RAGE induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20:742–752. https://doi.org/10.1681/ASN.2008050514
Author information
Authors and Affiliations
Contributions
TB: Conceived the idea, Wrote the article; ES and AS: Review of Literature; IK: Revision and Editing; RA: Drafting and alignment; GP and MK: Figure Work; RK and SB: Proof Read.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Behl, T., Sharma, E., Sehgal, A. et al. Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs. Mol Biol Rep 48, 1869–1881 (2021). https://doi.org/10.1007/s11033-020-06130-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06130-x