Abstract
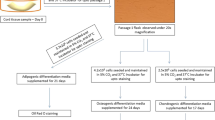
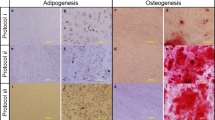
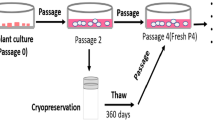
Mesenchymal stem cells (MSC) differentiate into different cell types and have immunomodulatory and paracrine effects. Cryopreservation of umbilical cord tissue as a source of MSC is very promising for regenerative medicine. We aim to evaluate a protocol for cryopreserving this tissue sectioned into small fragments with viable MSC. A total of 723 samples were frozen, thawed and cultured to obtain primary cultures of MSC. These were followed until 90–100% confluence and flow cytometric analysis were performed to confirm the mesenchymal phenotype. Samples in which protocol alterations at the collection of the samples were reported, were excluded for microbial contamination analysis leaving a total of 634 samples composed of 181 vaginal and 453 cesarean births. All cultures reach confluence with a media of 22.57 days and 97% in 28 or fewer days. Evaluated cultures showed low percentage of CD45+ and high of CD73 and CD90. Eight samples were subcultured 4 or 5 times and differentiated to chondrocytes and osteocytes to test differentiation potential with positive results. Umbilical cord tissue collections showed similar microbial profile and risk factors to those reported of umbilical cord blood collections, but with higher contamination frequencies. Cryopreserved tissue samples had viable cells that can be expanded without losing differentiation potential. Higher contamination frequencies compared to umbilical cord blood collection are not surprising, however, microbial load and survival of microorganisms to cryopreservation are expected to be lower.


Similar content being viewed by others
Change history
19 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11033-021-06206-2
References
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9:641–650. https://doi.org/10.1002/jor.1100090504
Caplan AI (2017) Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6:1445–1451. https://doi.org/10.1002/sctm.17-0051
Secunda R, Vennila R, Mohanashankar AM et al (2015) Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology 67:793–807. https://doi.org/10.1007/s10616-014-9718-z
Li T, Xia M, Gao Y et al (2015) Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther 15:1293–1306. https://doi.org/10.1517/14712598.2015.1051528
Ding D-C, Chang Y-H, Shyu W-C, Lin S-Z (2015) Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant 24:339–347. https://doi.org/10.3727/096368915X686841
Brown K, Rao M, Brown H (2019) The future state of newborn stem cell banking. J Clin Med 8:117. https://doi.org/10.3390/jcm8010117
Harris DT (2016) Banking of adipose- and cord tissue-derived stem cells: Technical and regulatory issues. In: Advances in experimental medicine and biology. Springer, New York, pp 147–154
Skiles ML, Brown KS, Tatz W et al (2018) Quantitative analysis of composite umbilical cord tissue health using a standardized explant approach and an assay of metabolic activity. Cytotherapy 20:564–575. https://doi.org/10.1016/j.jcyt.2018.01.001
Shimazu T, Mori Y, Takahashi A et al (2015) Serum- and xeno-free cryopreservation of human umbilical cord tissue as mesenchymal stromal cell source. Cytotherapy 17:593–600. https://doi.org/10.1016/j.jcyt.2015.03.604
Choudhery MS, Michael Badowski AM, DTH (2013) Utility of cryopreserved umbilical cord tissue for regenerative medicine. Curr Stem Cell Res Ther 8:370–380
Yong KW, Choi JR, Wan Safwani WKZ (2016) Biobanking of human mesenchymal stem cells: future strategy to facilitate clinical applications. In: Advances in experimental medicine and biology. Springer, New York, pp 99–110
Galipeau J, Sensébé L (2018) Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22:824–833
Banitalebi Dehkordi M, Madjd Z, Chaleshtori MH et al (2016) A simple, rapid, and efficient method for isolating mesenchymal stem cells from the entire umbilical cord. Cell Transplant 25:1287–1297. https://doi.org/10.3727/096368915X688911
Lin CS, Ning H, Lin G, Lue TF (2012) Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 14:1159–1163
Margossian T, Reppel L, Makdissy N et al (2012) Mesenchymal stem cells derived from Wharton’s jelly: comparative phenotype analysis between tissue and in vitro expansion. Biomed Mater Eng 22:243–254. https://doi.org/10.3233/BME-2012-0714
Magatti M, Pianta S, Silini A, Parolini O (2016) Isolation, culture, and phenotypic characterization of mesenchymal stromal cells from the amniotic membrane of the human term placenta. In: Methods in molecular biology. Humana Press Inc., Totowa, pp 233–244
Mennan C, Garcia J, Roberts S et al (2019) A comprehensive characterisation of large-scale expanded human bone marrow and umbilical cord mesenchymal stem cells. Stem Cell Res Ther 10:99. https://doi.org/10.1186/s13287-019-1202-4
Marupanthorn K, Tantrawatpan C, Kheolamai P et al (2017) Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int J Mol Med 39:654–662. https://doi.org/10.3892/ijmm.2017.2872
Gharravi AM (2019) Encapsulated explant in novel low shear perfusion bioreactor improve cell isolation, expansion and colony forming unit. Cell Tissue Bank. https://doi.org/10.1007/s10561-019-09749-8
Clark P, Trickett A, Stark D, Vowels M (2012) Factors affecting microbial contamination rate of cord blood collected for transplantation. Transfusion 52:1770–1777. https://doi.org/10.1111/j.1537-2995.2011.03507.x
Clark P, Trickett A, Saffo S, Stark D (2014) Effects of cryopreservation on microbial-contaminated cord blood. Transfusion 54:532–540. https://doi.org/10.1111/trf.12323
Bello-López JM, Noguerón-Silva J, Castañeda-Sánchez JI, Rojo-Medina J (2015) Molecular characterization of microbial contaminants isolated from umbilical cord blood units for transplant. Braz J Infect Dis. https://doi.org/10.1016/j.bjid.2015.07.005
Acknowledgments
We are grateful to the staff of Protectia Stem Cells Bank from Cordoba Argentina for sharing their mesenchymal stem cells cryopreservation advances used as a starting point for the optimized protocol presented in this work.
Author information
Authors and Affiliations
Contributions
R.D, M.A and F.S.D contributed to the design and implementation of the research, analysis of the results and writing of the manuscript. R.D designed and performed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report.
Ethical approval
All procedures performed with human samples were in accordance with the ethical standards of the research committee (CEIC, Comité de Etica en Investigación Clínica, http://www.comitedeeticaceic.com.ar/) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors names were interchanged in the original article. They should read Diego Raffo, Andrea Maglioco, and Diego Fernandez Sasso. The original article has been corrected.
Rights and permissions
About this article
Cite this article
Raffo, D., Maglioco, A. & Fernandez Sasso, D. A protocol for umbilical cord tissue cryopreservation as a source of mesenchymal stem cells. Mol Biol Rep 48, 1559–1565 (2021). https://doi.org/10.1007/s11033-020-06079-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06079-x