Abstract
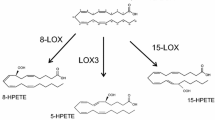
Neuroinflammation is well established biomarker for the major neurodegenerative like Alzheimer’s disease (AD) and Parkinson’s disease (PD). Cytokines/chemokines excite phospholipase A2 and cyclooxygenases (COX), facilitating the release of arachidonic acid (AA) and docosahexaenoic acid (DHA) from membrane glycerophospholipids, in which the former is oxidized to produce pro-inflammatory eicosanoids (prostaglandins, leukotrienes and thromboxane’s), which intensify the neuroinflammatory events in the brain. Similarly, resolvins and neuroprotectins are the metabolized products of docosahexaenoic acid, which exert an inhibitory effect on the production of eicosanoids. Furthermore, an oxidized product of arachidonic acid, lipoxin, is generated via 5-lipoxygenase (5-LOX) pathway, and contributes to the resolution of inflammation, along with anti-inflammatory actions. Moreover, DHA and its lipid mediators inhibit neuroinflammatory responses by blocking NF-κB, inhibiting eicosanoid production, preventing cytokine secretion and regulating leukocyte trafficking. Various epidemiological studies reported, elevated levels of COX-2 enzyme in patients with AD and PD, indicating its role in progression of the disease. Similarly, enhanced levels of 5-LOX and 12/15-LOX in PD models represent their role brain disorders, where the former is expressed in AD patients and the latter exhibits it involvement in PD. The present review elaborates the role of AA, DHA, eicosanoids and docosanoids, along with COX and LOX pathway which provides an opportunity to the researchers to understand the role of these lipid mediators in neurological disorders (AD and PD). The information gathered from the review will aid in facilitating the development of appropriate therapeutic options targeting COX and LOX pathway.



Similar content being viewed by others
References
Wilson CJ, Finch CE, Cohen HJ (2002) Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc 50:2041–2056
Joffre C, Grégoire S, Smedt VE, Acar N, Bretillon L, Nadjar A, Layé S (2016) Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot Essent Fatty Acids 114:1–10
Joffre C (2019) Polyunsaturated fatty acid metabolism in the brain and brain cells. IntechOpen. https://doi.org/10.5772/intechopen.88232
Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR (1993) Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest 91(2):651–660
Bazinet RP, Layé S (2014) Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15(12):771–785
Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K (2000) Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192(8):1197–1204
Rey C, Delpech JC, Madore C, Nadjar A, Greenhalgh AD, Amadieu C, Aubert A, Pallet V, Vaysse C, Layé S, Joffre C (2019) Dietary n-3 long chain PUFA supplementation promotes a proresolvingoxylipin profile in the brain. Brain Behav Immun 76:17–27
Shalini SM, Ho CFY, Ng YK, Tong JX, Ong ES, Herr DR, Dawe GS, Ong WY (2018) Distribution of Alox15 in the rat brain and its role in prefrontal cortical resolvin D1 formation and spatial working memory. Mol Neurobiol 55(2):1537–1550
Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VMY (2004) 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol 164(5):1655–1662
Sun L, Xu YW, Han J, Liang H, Wang N, Cheng Y (2015) 12/15-Lipoxygenase metabolites of arachidonic acid activatePPARgamma: a possible neuroprotective effect in ischemic brain. J Lipid Res 56(3):502–514
Bystrom J, Wray JA, Sugden MC, Holness MJ, Swales KE, Warner TD, Edin ML, Zeldin DC, Gilroy DW, Bishop-Bailey D (2011) Endogenous epoxygenases are modulators of monocyte/macrophage activity. PLoS One 6(10):e26591
Farooqui AA, Horrocks LA, Farooqui T (2007) Modulation of inflammation in brain: a matter of fat. J Neurochem 101:577–599
Farooqui AA, Horrocks LA, Farooqui T (2000b) Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem Phys Lipids 106:1–29
Farooqui AA, Rammohan KW, Horrocks LA (1989) Isolation, characterization and regulation of diacylglycerol lipases from bovine brain. Ann N Y Acad Sci 559:25–36
Bochkov VN, Leitinger N (2003) Anti-inflammatory properties of lipid oxidation products. J Mol Med 81:613–626
Lee H, Villacreses NE, Rapoport SI, Rosenberger TA (2004) In vivo imaging detects a transient increase in brain arachidonic acid metabolism: a potential marker of neuroinflammation. J Neurochem 91:936–945
Kolko M, Rodriguez de Turco EB, Diemer NH, Bazan NG (2002) Secretory phospholipase A2-mediated neuronal cell death involves glutamate ionotropic receptors. NeuroReport 13:1963–1966
Sun GY, Xu J, Jensen MD, Simonyi A (2004b) Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res 45:205–213
Moses GSD, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY (2006) Secretory PLA2-IIA: a new inflammatory factor for Alzheimer’s disease. J Neuroinflammation 3:28–38
Phillis JW, Horrocks LA, Farooqui AA (2006) Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev 52:201–243
Gilroy DW, Newson J, Sawmynaden PA, Willoughby DA, Croxtall JD (2004) A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J 18:489–498
Zhang J, Rivest S (2001) Anti-inflammatory effects of prostaglandin E2 in the central nervous system in response to brain injury and circulating lipopolysaccharide. J Neurochem 76:855–864
Chiang N, Arita M, Serhan CN (2005) Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids 73:163–177
Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J (2006) Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med 12:330–334
Kantarci A, Van Dyke TE (2003) Lipoxins in chronic inflammation. Crit Rev Oral Biol Med 14:4–12
Fam SS, Morrow JD (2003) The isoprostanes: unique products of arachidonic acid oxidation—a review. Curr Med Chem 10:1723–1740
Serhan CN, Arita M, Hong S, Gotlinger K (2004) Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 39:1125–1132
Gronert K (2005) Lipoxins in the eye and their role in wound healing. Prostaglandins Leukot Essent Fatty Acids 73:221–229
Czapski GA, Czubowicz K, Strosznajder JB, Strosznajder RP (2016) The lipoxygenases: their regulation and implication in Alzheimer’s disease. Neurochem Res 41:243–257. https://doi.org/10.1007/s11064-015-1776-x
Breydo L, Wu JW, Uversky V (2012) Α-synuclein misfolding and Parkinson's disease. Biochim Biophys Acta 1822(2):261–285
Ton TG, Jain S, Biggs ML, Thacker EL, Strotmeyer ES, Boudreau R, Newman AB, Longstreth WT Jr, Checkoway H (2012) Markers of inflammation in prevalent and incident Parkinson’s disease in the Cardiovascular Health Study. Parkinsonism Relat Disord 18(3):274–278
Yamamoto S, Suzuki H, Ueda N (1997) Arachidonate 12-lipoxygenases. Prog Lipid Res 36(1):23–41
Schmitz G, Ecker J (2008) The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 47(2):147–155
Amandi-Burgermeister E, Tibes U, Kaiser BM, Friebe WG, Scheuer WV (1997) Suppression of cytokine synthesis, integrin expression and chronic inflammation by inhibitors of cytosolic phospholipase A2. Eur J Pharmacol 326(2–3):237–250
Sorensen HN, Treuter E, Gustafsson JA (1998) Regulation of peroxisome proliferator-activated receptors. Vitam Horm 54:121–166
Hampson AJ, Grimaldi M (2002) 12-Hydroxyeicosatetrenoate (12-HETE) attenuates AMPA receptor-mediated neurotoxicity: evidence for a G-protein-coupled HETE receptor. J Neurosci 22(1):257–264
Chinnici CM, Yao Y, Ding T, Funk CD, Pratico D (2005) Absence of 12/15 lipoxygenase reduces brain oxidative stress in apolipoprotein E-deficient mice. Am J Pathol 167(5):1371–1377
Zafiriou MP, Deva R, Ciccoli R, Siafaka-Kapadai A, Nigam S (2007) Biological role of hepoxilins: upregulation of phospholipid hydroperoxide glutathione peroxidase as a cellular response to oxidative stress? Prostaglandins Leukot Essent Fatty Acids 77(3–4):209–215
Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, Rosenberg PA (2004) Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci 24(47):10616–10627
Giannopoulos PF, Joshi YB, Chu J, Pratico D (2013) The 12-15-lipoxygenase is a modulator of Alzheimer’s-related tau pathology in vivo. Aging Cell 12(6):1082–1090
Strosznajder JB, Cieslik M, Cakala M, Jesko H, Eckert A, Strosznajder RP (2011) Lipoxygenases and poly(ADP-ribose) polymerase in amyloid beta cytotoxicity. Neurochem Res 36(5):839–848
Lebeau A, Esclaire F, Rostene W, Pelaprat D (2001) Baicalein protects cortical neurons from beta-amyloid (25–35) induced toxicity. NeuroReport 12(10):2199–2202
Aisen PS (2002) The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. Lancet Neurol 1(5):279–284
Lukiw WJ, Bazan NG (2006) Survival signalling in Alzheimer’s disease. Biochem Soc Trans 34(Pt 6):1277–1282
Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG (2011) Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS One 6(1):e15816
Van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH (2006) Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37:3014–3018
Li Y, Maher P, Schubert DA (1997) Role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 19:453–463
Canals S, Casarejos MJ, De Bernardo S, Rodriguez-Martin E, Mena MA (2003) Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. J Biol Chem 278:21542–21549
Charlier C, Michaux C (2003) Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem 38(7–8):645–659
Manev H et al (2011) Cyclooxygenases and 5-lipoxygenase in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 35(2):315–319
Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D (2008) 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J 22(4):1169–1178
Clària J, Serhan CN (1995) Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A 92(21):9475–9479
Chu LS, Fang SH, Zhou Y, Yin YJ, Chen WY, Li JH, Sun J, Wang ML, Zhang WP, Wei EQ (2010) Minocycline inhibits 5-lipoxygenase expression and accelerates functional recovery in chronic phase of focal cerebral ischemia in rats. Life Sci 86(5–6):170–177
Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P (1996) COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A 93:2317–2321
Vane JR, Bakhle YS, Botting RM (1998) Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120
Oka A, Takashima S (1997) Induction of cyclo-oxygenase 2 in brains of patients with Down’s syndrome and dementia of Alzheimer type: specific localization in affected neurones and axons. Neuroreport 8:1161–1164
Braak H, Braak E (1991) Neuropathological stageing of Alzheimerrelated changes. Acta Neuropathol 82:239–259
Ho L, Purohit D, Haroutunian V, Luterman JD, Willis F, Naslund J, Buxbaum JD, Mohs RC, Aisen PS, Pasinetti GM (2001) Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol 58:487–492
Hoozemans JJ, Veerhuis R, Rozemuller AJ, Arendt T, Eikelenboom P (2004) Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol Dis 15:492–499
Vincent I, Jicha G, Rosado M, Dickson DW (1997) Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci 17:3588–3598
Liu DX, Greene LA (2001) Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res 305:217–228
Gouras GK, Almeida CG, Takahashi RH (2005) Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging 26:1235–1244
Hoozemans JJM et al (2008) Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr Pharm Des 14:1419–1427
Bauer MK, Lieb K, Schulze-Osthoff K, Berger M, Gebicke-Haerter PJ, Bauer J et al (1997) Expression and regulation of cyclooxygenase-2 in rat microglia. Eur J Biochem 243:726–731
Alvarez V, González P, Corao AI, Menéndez M, Lahoz CH, Martínez C, Calatayud M, Morales B, Coto E (2008) The Sp1/Egr1-tandem repeat polymorphism in the 5-lipoxygenase gene promoter is not associated with late onset Alzheimer disease. Alzheimer Dis Assoc Disord 22(2):177–180
Aïd S, Bosetti F (2007) Gene expression of cyclooxygenase-1 and Ca(2+)-independent phospholipase A(2) is altered in rat hippocampus during normal aging. Brain Res Bull 73(1–3):108–113
Fujimi K, Noda K, Sasaki K, Wakisaka Y, Tanizaki Y, Iida M, Kiyohara Y, Kanba S, Iwaki T (2007) Altered expression of COX-2 in subdivisions of the hippocampus during aging and in Alzheimer’s disease: the Hisayama Study. Dement Geriatr Cogn Disord 23(6):423–431
Kitamura Y, Shimohama S, Koike H, KakimuraJi Y et al (1999) Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer's disease brains. Biochem Biophys Res Commun 254(3):582–586
Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, Kaufmann WE, Vehmas A, Andreasson KI (2006) Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer's disease in a sex-dimorphic pattern. Neuroscience 141(3):1149–1162
Kukar T, Golde TE (2008) Possible mechanisms of action of NSAIDs and related compounds that modulate gamma-secretase cleavage. Curr Top Med Chem 8(1):47–53
Mirza B, Hadberg H, Thomsen P, Moos T (2009) The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience 95:425–432
De Simone R, Ajmone-Cat MA, Minghetti L (2004) Atypical antiinflammatory activation of microglia induced by apoptotic neurons: possible role of phosphatidylserine-phosphatidylserine receptor interaction. Mol Neurobiol 29:197–212
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A (2001) CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci 4:702–710
Beach TG, Sue LI, Walker DG, Lue LF, Connor DJ, Caviness JN, Sabbagh MN, Adler CH (2007) Marked microglial reaction in normal aging human substantianigra: correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathol 114:419–424
Mcgeer PL, Itagaki S, Boyes BE, Mcgeer EG (1998) Reactive microglia are positive for Hla-Dr in the SubstantiaNigra of Parkinsons and Alzheimers-disease brains. Neurology 38:1285–1291
Bartels AL, Leenders KL (2010) Cyclooxygenase and neuroinflammation in Parkinson’s disease neurodegeneration. Curr Neuropharmacol 8:62–68
Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D, Vila M, Jackson-Lewis V, Przedborski S (2003) Pathogenic role of glial cells in Parkinson’s disease. Mov Disord 18:121–129
Dehmer T (2004) Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem 88:494–501
Minghetti L (2004) Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol 63:901–910
Knott C, Stern G, Wilkin GP (2000) Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci 16:724–739
Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O (2004) Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson’s disease. J Neuroinflammation 1:6
Sriram K, Miller DB, O'Callaghan JP (2006) Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem 96:706–718
Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100:1375–1386
Wang TG, Pei Z, Zhang W, Liu B, Langenbach R, Lee C, Wilson B, Reece JM, Miller DS, Hong JS (2005) MPP+- induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J 19:134–1136
Bartels AL, Willemsen ATM, Kortekaas R, de Jong BM, de Vries R, de Klerk O, van Oostrom JCH, Portman A, Leenders KL (2008) Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm 115:1001–1009
Bauer B, Anika MSH, Pekcec A, Toellner K, Miller DSPH (2008) Seizure-induced up-regulation of P glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol 73:1444–1453
De Vries EFJ, Doorduin J, Vellinga NAR, Waarde AV (2008) Can celecoxib affect Pglycoprotein-mediated drug efflux? A microPET study. Nucl Med Biol 35:459–466
Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R (2005) Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Rev 48:251–256
McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K (2004) Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci 24:257–268
Colville-Nash PR, Gilroy DW (2001) Potential adverse effects of cyclooxygenase-2 inhibition: evidence from animal models of inflammation. Bio Drugs 15:1–9
Aid S, Langenbach R, Bosetti F (2008) Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation 5:17
Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A (2005) Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol 58:963–967
Wahner AD, Bronstein JM, Bordelon YM, Ritz B (2007) Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology 69:1836–1842
Bower JH, Maraganore DM, Peterson BJ, Ahlskog JE, Rocca WA (2006) Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: a case-control study. Neurology 67:494–496
Ton TG, Heckbert SR, Longstreth WT Jr, Rossing MA, Kukull WA, Franklin GM, Swanson PD, Smith-Weller T, Checkoway H (2006) Nonsteroidal anti-inflammatory drugs and risk of Parkinson’s disease. Mov Disord 21:964–969
Etminan M, Carleton BC, Samii A (2008) Non-steroidal anti-inflammatory drug use and the risk of Parkinson disease: a retrospective cohort study. J Clin Neurosci 15:576–577
Minutoli L, Marini H, Rinaldi M, Bitto A, Irrera N, Pizzino G, Pallio G, Calò M, Adamo EB, Trichilo V, Interdonato M, Galfo F, Squadrito F, Altavilla D (2015) A dual inhibitor of cyclooxygenase and 5-lipoxygenase protects against kainic acid-induced brain injury. Neuromolecular Med 17(2):192–201
Bitto A, Giuliani D, Pallio G, Irrera N, Vandini E, Canalini F, Zaffe D, Ottani A, Minutoli L, Rinaldi M, Guarini S, Squadrito F, Altavilla D (2017) Effects of COX1-2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm Res 66(5):389–398
Acknowledgements
The authors would like to thank Chitkara College of Pharmacy, Chitkara University, Punjab, India for providing basic facilities for completion of the article.
Funding
Authors did not receive any funding for this completing this review article.
Author information
Authors and Affiliations
Contributions
AK: Conceived the idea and wrote the first Draft, TB: Improved the article, SJ: Review of Literature, IK: Writing and improvement of article, AS: Proof Read, PK: Proof Read and improvement of article.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Behl, T., Jamwal, S. et al. Exploring the molecular approach of COX and LOX in Alzheimer’s and Parkinson’s disorder. Mol Biol Rep 47, 9895–9912 (2020). https://doi.org/10.1007/s11033-020-06033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06033-x