Abstract
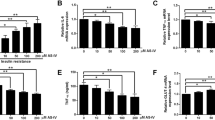
Mitochondrial dysfunction plays a crucial role in the central pathogenesis of insulin resistance and type 2 diabetes mellitus. Macrophages play important roles in the pathogenesis of insulin resistance. Lauric acid is a 12-carbon medium chain fatty acid (MCFA) found abundantly in coconut oil or palm kernel oil and it comes with multiple beneficial effects. This research objective was to uncover the effects of the lauric acid on glucose uptake, mitochondrial function and mitochondrial biogenesis in insulin-resistant macrophages. THP-1 monocytes were differentiated into macrophages and induce insulin resistance, before they were treated with increasing doses of lauric acid (5 μM, 10 μM, 20 μM, and 50 μM). Glucose uptake assay, cellular ROS and ATP production assays, mitochondrial content and membrane potential assay were carried out to analyse the effects of lauric acid on insulin resistance and mitochondrial biogenesis in the macrophages. Quantitative RT-PCR (qRT-PCR) and western blot analysis were also performed to determine the expression of the key regulators. Insulin-resistant macrophages showed lower glucose uptake, GLUT-1 and GLUT-3 expression, and increased hallmarks of mitochondrial dysfunction. Interestingly, lauric acid treatment upregulated glucose uptake, GLUT-1 and GLUT-3 expressions. The treatment also restored the mitochondrial biogenesis in the insulin-resistant macrophages by improving ATP production, oxygen consumption, mitochondrial content and potential, while it promoted the expression of mitochondrial biogenesis regulator genes such as TFAM, PGC-1α and PPAR-γ. We show here that lauric acid has the potential to improve insulin sensitivity and mitochondrial dysregulation in insulin-resistant macrophages.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
World Health Organization (2016) Global report on diabetes. WHO, Geneva
The Emerging Risk Factors Collaboration (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222. https://doi.org/10.1016/S0140-6736(10)60484-9
Letchuman GR, Wan Nazaimoon WM, Wan Mohamad WB et al (2010) Prevalence of diabetes in the Malaysian National Health Morbidity Survey III 2006. Med J Malaysia 65:173–179
Piťhová P, Štechová K, Piťha J et al (2016) Determinants of preclinical atherosclerosis are different in type 1 and type 2 diabetic women. Physiol Res 65:219–228. https://doi.org/10.33549/physiolres.933019
Carvalho E, Kotani K, Peroni OD, Kahn BB (2005) Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab 289:551–561. https://doi.org/10.1152/ajpendo.00116.2005
Schlößer HA, Drebber U, Urbanski A et al (2017) Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer 20:83–91. https://doi.org/10.1007/s10120-015-0577-x
Poupel L, Combadière C (2010) Athérosclérose: sur la piste des chimiokines. Biol Aujourdhui 204:285–293. https://doi.org/10.1051/jbio/2010026
Liang CP, Han S, Senokuchi T, Tall AR (2007) The macrophage at the crossroads of insulin resistance and atherosclerosis. Circ Res 100:1546–1555. https://doi.org/10.1161/CIRCRESAHA.107.152165
Madamanchi NR, Runge MS (2007) Mitochondrial dysfunction in atherosclerosis. Circ Res 100:460–473. https://doi.org/10.1161/01.RES.0000258450.44413.96
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312. https://doi.org/10.1042/BJ20110162
Hao J, Shen W, Yu G et al (2010) Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J Nutr Biochem 21:634–644. https://doi.org/10.1016/j.jnutbio.2009.03.012
Lira VA, Benton CR, Yan Z, Bonen A (2010) PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 299. https://doi.org/10.1152/ajpendo.00755.2009
Picca A, Lezza AMS (2015) Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions. Useful insights from aging and calorie restriction studies. Mitochondrion 25:67–75. https://doi.org/10.1016/j.mito.2015.10.001
Dayrit FM (2015) The properties of lauric acid and their significance in coconut oil. J Am Oil Chem Soc 92:1–15. https://doi.org/10.1007/s11746-014-2562-7
Lieberman S, Enig MG, Preuss HG (2006) A review of monolaurin and lauric acid: natural virucidal and bactericidal agents. Altern Complement Ther 12:310–314. https://doi.org/10.1089/act.2006.12.310
Alves NFB, de Queiroz TM, de Almeida TR et al (2017) Acute treatment with lauric acid reduces blood pressure and oxidative stress in spontaneously hypertensive rats. Basic Clin Pharmacol Toxicol 120:348–353. https://doi.org/10.1111/bcpt.12700
Huang WC, Tsai TH, Te Chuang L et al (2014) Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: a comparative study with lauric acid. J Dermatol Sci 73:232–240. https://doi.org/10.1016/j.jdermsci.2013.10.010
Bobiński R, Wyszomirski M, Machnickam A et al (2019) The effect of lauric acid on pathogens colonizing the burn wound: a pilot study. Altern Ther Health Med
Ong MHL, Wong HK, Tengku-Muhammad TS et al (2019) Pro-atherogenic proteoglycanase ADAMTS-1 is down-regulated by lauric acid through PI3K and JNK signaling pathways in THP-1 derived macrophages. Mol Biol Rep 46:2631–2641. https://doi.org/10.1007/s11033-019-04661-6
Daigneault M, Preston JA, Marriott HM et al (2010) The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 5. https://doi.org/10.1371/journal.pone.0008668
Rui L, Aguirre V, Kim JK et al (2001) Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107:181–189. https://doi.org/10.1172/JCI10934
Aranda PS, Lajoie DM, Jorcyk CL (2012) Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis 33:366–369. https://doi.org/10.1002/elps.201100335
Anghebem-Oliveira MI, Martins BR, Alberton D et al (2017) Type 2 diabetes-associated genetic variants of FTO, LEPR, PPARg, and TCF7L2 in gestational diabetes in a Brazilian population. Arch Endocrinol Metab 61:238–248. https://doi.org/10.1590/2359-3997000000258
Miranda D, Jara C, Ibañez J et al (2016) PGC-1α-dependent mitochondrial adaptation is necessary to sustain IL-2-induced activities in human NK cells. Mediators Inflamm 2016. https://doi.org/10.1155/2016/9605253
Zhu Y, Chen G, Chen L et al (2014) Monitoring mitophagy in mammalian cells. In: Methods in enzymology, 1st edn. Elsevier Inc., pp 39–55
Fang P, Yu M, Zhang L et al (2017) Baicalin against obesity and insulin resistance through activation of AKT/AS160/GLUT4 pathway. Mol Cell Endocrinol 448:77–86. https://doi.org/10.1016/j.mce.2017.03.027
Arnold SE, Arvanitakis Z, Macauley-Rambach SL et al (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14:168–181. https://doi.org/10.1038/nrneurol.2017.185
Han T, Lv Y, Wang S et al (2019) PPARγ overexpression regulates cholesterol metabolism in human L02 hepatocytes. J Pharmacol Sci 139:1–8. https://doi.org/10.1016/j.jphs.2018.09.013
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH et al (2007) Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447:1116–1120. https://doi.org/10.1038/nature05894
Rial SA, Ravaut G, Malaret TB et al (2018) Hexanoic, octanoic and decanoic acids promote basal and insulin-induced phosphorylation of the Akt-MTOR axis and a balanced lipid metabolism in the HEPG2 hepatoma cell line. Molecules 23:1–16. https://doi.org/10.3390/molecules23092315
Evans AMSNHRM (2013) PPAR gamma, the good, the bad & the future. Nat Med 19. https://doi.org/10.1038/nm.3159.PPAR
Liberato MV, Nascimento AS, Ayers SD et al (2012) Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) γ activators and Pan-PPAR partial agonists. PLoS One 7:1–10. https://doi.org/10.1371/journal.pone.0036297
Mailloux RJ, Harper ME (2011) Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51:1106–1115. https://doi.org/10.1016/j.freeradbiomed.2011.06.022
Wang Y, Tabas I (2014) Emerging roles of mitochondria ROS in atherosclerotic lesions: causation or association? J Atheroscler Thromb 21:381–390. https://doi.org/10.5551/jat.23929
Tuominen A, Miller YI, Hansen LF et al (2006) A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol 26:2096–2102. https://doi.org/10.1161/01.ATV.0000233333.07991.4a
Szendroedi J, Phielix E, Roden M (2012) The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 8:92–103. https://doi.org/10.1038/nrendo.2011.138
Montgomery MK, Turner N (2015) Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 4. https://doi.org/10.1530/EC-14-0092
Jing L, He MT, Chang Y et al (2015) Coenzyme Q10 protects astrocytes from ROS-induced damage through inhibition of mitochondria-mediated cell death pathway. Int J Biol Sci 11:59–66. https://doi.org/10.7150/ijbs.10174
Fang WJ, Wang CJ, He Y et al (2018) Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol Sin 39:59–73. https://doi.org/10.1038/aps.2017.50
Urbanová M, Mráz M, Ďurovcová V et al (2017) The effect of very-low-calorie diet on mitochondrial dysfunction in subcutaneous adipose tissue and peripheral monocytes of obese subjects with type 2 diabetes mellitus. Physiol Res 66:811–822. https://doi.org/10.33549/physiolres.933469
Wilson DF (2017) Oxidative phosphorylation: regulation and role in cellular and tissue metabolism. J Physiol 595:7023–7038. https://doi.org/10.1113/JP273839
Rabøl R, Højberg PMV, Almdal T et al (2009) Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab 94:1372–1378. https://doi.org/10.1210/jc.2008-1475
Sergi D, Naumovski N, Heilbronn LK et al (2019) Mitochondrial (dys)function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front Physiol 10. https://doi.org/10.3389/fphys.2019.00532
Mitra R, Nogee DP, Zechner JF et al (2012) The transcriptional coactivators, PGC-1α and β, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J Mol Cell Cardiol 52:701–710. https://doi.org/10.1016/j.yjmcc.2011.10.010
Vernochet C, Damilano F, Mourier A et al (2014) Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J 28:4408–4419. https://doi.org/10.1096/fj.14-253971
Kochikuzhyil BM, Devi K, Fattepur SR (2010) Effect of saturated fatty acid-rich dietary vegetable oils on lipid profile, antioxidant enzymes and glucose tolerance in diabetic rats. Indian J Pharmacol 42:142–145. https://doi.org/10.4103/0253-7613.66835
Sellin J, Wingen C, Gosejacob D et al (2018) Dietary rescue of lipotoxicity-induced mitochondrial damage in Peroxin19 mutants. PLoS Biol 16:1–24. https://doi.org/10.1371/journal.pbio.2004893
Montgomery MK, Osborne B, Brown SHJ et al (2013) Contrasting metabolic effects of medium-versus long-chain fatty acids in skeletal muscle. J Lipid Res 54:3322–3333. https://doi.org/10.1194/jlr.M040451
Lee JH, Zhang Y, Zhao Z et al (2017) Intracellular ATP in balance of pro- and anti-inflammatory cytokines in adipose tissue with and without tissue expansion. Int J Obes 41:645–651. https://doi.org/10.1038/ijo.2017.3
Miyagawa Y, Mori T, Goto K et al (2018) Intake of medium-chain fatty acids induces myocardial oxidative stress and atrophy. Lipids Health Dis 17:1–7. https://doi.org/10.1186/s12944-018-0908-0
Acknowledgements
This work was supported by Malaysia’s Ministry of Higher Education’s Fundamental Research Grant Scheme (FRGS) (FRGS/1/2016/SKK06/UTAR/02/1).
Funding
This work was supported by Malaysia’s Ministry of Higher Education’s Fundamental Research Grant Scheme (FRGS) (FRGS/1/2016/SKK06/UTAR/02/1).
Author information
Authors and Affiliations
Contributions
YY Tham carried out the experiment and data analyses/interpretation, and wrote the manuscript. CH Chew was the principal investigator and contributed to the study design as well as interpretation of the findings. QC Choo and TM Tengku Muhammad were the co-investigators of the grants.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tham, Y.Y., Choo, Q.C., Muhammad, T.S.T. et al. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol Biol Rep 47, 9595–9607 (2020). https://doi.org/10.1007/s11033-020-06019-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06019-9