Abstract
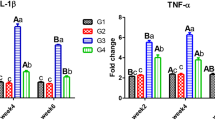
High ammonia can inhibit the survival and growth, and even cause mortality of razor clam (S. constricta). The accumulation of ammonia to lethal concentrations in some invertebrates may be partially prevented by converting some of the ammonia into glutamine (Gln). Glutamine dehydrogenase (GDH) and glutamine synthetase (GS) have been widely implicated a central role in response to ammonia stress. However, the molecular and physiological response of GDH and GS to ammonia alterations has not yet been determined in clams. To investigate the possible participatory role of GDH and GS genes in altered ammonia conditions, we have cloned their gene sequences and examined the mRNA expression and western blotting under ammonia exposure in S. constricta (ScGDH and ScGS), and detected the levels of GS and GDH, and the content of glutamate (Glu) and Gln. The full-length cDNA of ScGDH was 3924 bp, with a 1629 bp open reading frame (ORF) encoding a 542 amino-acid polypeptide. The complete cDNA sequence for ScGS had 2739 bp with an ORF of 1110 bp encoding 369 amino acids. To investigate ammonia detoxification strategies, the clams were exposed to ammonia for 96 h at four different concentrations (0, 100, 140, and 180 mg/L). Exposure to ammonia resulted in a significant increase of glutamate concentration and Gln in the haemocytes. GDH activity, GDH relative mRNA and protein expression, GS activity, GS relative mRNA and protein expression increased significantly and showed a pronounced time and dosage interaction in the liver. The results suggested that the protective strategies of Gln formation existed in S. constricta, which could convert ammonia to non- or less toxic nitrogenous compounds on the exposure of ammonia. Glutamate content in the haemocytes increased significantly, which is to ensure sufficient Glu to meet the needs for GS to catalyze the conversion of ammonia to Gln. We proposed that the induction of Glu synthesis-related genes and the subsequent formation of the active protein occurred in preparation for the increased capacity of the body to convert ammonia, into Gln. The results of this study suggested that GDH and GS play an important role in the synthesis of Gln, emphasizing, the protective strategies of Gln formation in S. constricta convert ammonia to nontoxic or less toxic nitrogenous compounds upon exposure to ammonia.





Similar content being viewed by others
References
Wang XR, Xue QG, Mao XW, Dong YH, Li CH, Lin ZH (2018) Two I84 family protease inhibitors from Chinese razor clams, Sinonovacula constricta, expressed in response to environmental challenges. Fish Shellfish Immunol 75:149–157
The ministry of agriculture and fishery of the People’s Republic of China (2018) Chinese fishery statistical yearbook 2018. China Agriculture Press, Beijing
Cheng CH, Ma HL, Su YL, Deng YQ, Feng J, Xie JW, Chen XL, Guo ZX (2019) Ammonia toxicity in the mud crab (Scylla paramamosain), the mechanistic insight from physiology to transcriptome analysiss. Ecotoxicol Environ Saf 179:9–16
Xiao J, Li QY, Tu JP, Chen XL, Chen XH, Liu QY, Liu H, Zhou XY, Zhao YZ, Wang HL (2019) Stress response and tolerance mechanisms of ammonia exposure based on transcriptomics and metabolomics in Litopenaeus vannamei. Ecotoxicol Environ Saf 180:491–500
Wright PA, Steele SL, Huitema A, Bernier NJ (2007) Induction of four glutamine synthetase genes in brain of rainbow trout in response to elevated environmental ammonia. J Exp Biol 210(16):2905–2911
Rodela TM, Esbaugh AJ, Weihrauch D, Veauvy CM, McDonald MD, Gilmour KM, Walsh PJ (2012) Revisiting the effects of crowding and feeding in the gulf toadfish, Opsanus beta: the role of rhesus glycoproteins in nitrogen metabolism and excretion. J Exp Biol 215(2):301–313
Si LJ, Pan LQ, Wang HD, Zhang X (2018) Identification of the role of Rh protein in ammonia excretion of swimming crab Portunus trituberculatus. J Exp Biol 221(20):1–12
Sinha AK, Diric M, Chan LP, Liew HJ, Kumar V, Blust R, Boeck GD (2012) Expression pattern of potential biomarker genes related to growth, ion regulation and stress in response to ammonia exposure, food deprivation and exercise in common carp (Cyprinus carpio). Aquat Toxicol 122–123(2):93–105
Cheng CH, Yang FF, Liao SA, Miao YT, Ye CX, Wang AL (2015) Effect of acute ammonia exposure on expression of GH/IGF axis genes GHR1, GHR2 and IGF-1 in pufferfish (Takifugu obscurus). Fish Physiol Biochem 41(2):495–507
Liang ZX, Liu R, Zhao DP, Wang LL, Sun MZ, Wang MQ, Song LS (2016) Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol 54:523–528
Zhang MZ, Li M, Wang RX, Qian YX (2018) Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish Immunol 79:313–320
Li M, Gong S, Li Q, Yuan L, Meng F (2016) Wang R. Ammonia toxicity induces glutamine accumulation, oxidative stress and immunosuppression in juvenile yellow catfish Pelteobagrus fulvidraco. Comp Biochem Physiol C 183–184:1–6
Lu X, Luan S, Dai P, Meng XH, Cao BX, Luo K, Kong J (2018) iTRAQ-based comparative proteome analysis for molecular mechanism of defense against acute ammonia toxicity in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 74:52–61
Schram E, Roques JAC, Abbink W, Spanings T, Vries PD, Bieman S, Vis HVD, Flik G (2010) The impact of elevated water ammonia concentration on physiology, growth and feed intake of African catfish (Clarias gariepinus). Aquaculture 306(1–4):108–115
Smutná M, Vorlová L, Svobodová Z (2002) Pathobiochemistry of ammonia in the internal environment of fish (Review). Acta Vet Brno 71(2):169–181
Tok CY, Chew SF, Ip YK (2011) Gene cloning and mRNA expression of glutamate dehydrogenase in the liver, brain, and intestine of the swamp eel, Monopterus albus (Zuiew), exposed to freshwater, terrestrial conditions, environmental ammonia, or salinity stress. Front Physiol 2(100):2
Braun MH, Perry SF (2010) Ammonia and urea excretion in the Pacific hagfish Eptatretus stoutii: evidence for the involvement of Rh and UT proteins. Comp Biochem Physiol A 157(4):405–415
Chew SF (2003) Urea synthesis in the African lungfish Protopterus dolloi – hepatic carbamoyl phosphate synthetase iii and glutamine synthetase are upregulated by 6 days of aerial exposure. J Exp Biol 206(20):3615–3624
Ip YK, Chew SF, Wilson JM, Randall DJ (2004) Defences against ammonia toxicity in tropical air-breathing fishes exposed to high concentrations of environmental ammonia: a review. J Comp Physiol B 174(7):565–575
Ip YK, Chew SF (2010) Ammonia production, excretion, toxicity, and defense in fish, a review. Front Physiol 1(134):1–20
Wang GH, Pan LQ, Ding YG (2014) Defensive strategies in response to environmental ammonia exposure of the sea cucumber Apostichopus japonicus: glutamine and urea formation. Aquaculture 432:278–285
Weihrauch D (2004) Ammonia excretion in aquatic and terrestrial crabs. J Exp Biol 207(26):4491–4504
Weihrauch D, Wilkie MP, Walsh PJ (2009) Ammonia and urea transporters in gills of fish and aquatic crustaceans. J Exp Biol 212(17):1716–1730
Liu SN, Pan LQ, Liu MQ, Yang LB (2014) Effects of ammonia exposure on nitrogen metabolism in gills and hemolymph of the swimming crab Portunus trituberculatus. Aquaculture 432:351–359
Qiu LG, Shi X, Yu SM, Han Q, Diao XP, Zhou HL (2018) Changes of ammonia-metabolizing enzyme activity and gene expression of two strains in shrimp Litopenaeus vannamei under ammonia stress. Front Physiol 9:211
Lim SL, Ip E, Jow A, Chew SF, Lim CB, Anderson PM, Ip YK (1999) The marble goby oxyeleotris marmoratus activates hepatic glutamine synthetase and detoxifies ammonia to glutamine during air exposure. J Exp Biol 202(3):237–245
Banerjee B, Koner D, Bhuyan G, Saha N (2018) Differential expression of multiple glutamine synthetase genes in air-breathing Magur catfish, Clarias magur and their induction under hyper-ammonia stress. Gene 671:85–95
Cooper AJL, Jeitner TM (2016) Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain. Biomolecules 6(2):16
Wang YR, Li E, Yu N, Wang XD, Cai CF, Tang BP, Chen LQ, Wormhoudt AV (2012) Characterization and expression of glutamate dehydrogenase in response to acute salinity stress in the Chinese mitten crab, Eriocheir sinensis. PLoS One 7(5):e37316
Treberg JR, Banh S, Pandey U, Weihrauch D (2014) Intertissue differences for the role of glutamate dehydrogenase in metabolism. Neurochem Res 39(3):516–526
Gaspar C, Silva-Marrero JI, Fàbregas A, Miñarrob M, Ticób JR, Baanante IV, Metón I (2018) Administration of chitosan-tripolyphosphate-DNA nanoparticles to knockdown glutamate dehydrogenase expression impairs transdeamination and gluconeogenesis in the liver. J Biotechnol S0168–1656(18):30629–30621
Wang P, Tan Z, Guan L, Tang S, Zhou C, Han X, Kang J, He Z (2015) Ammonia and amino acids modulates enzymes associated with ammonia assimilation pathway by ruminal microbiota in vitro. Livest Sci 178:130–139
Ding YT, Zang XN, Shi JW, Hou LL, He BX, Dong MM, Cong XM, Cao XX, Liu Z, Song XW, Huang XY, Zhang XC (2019) cDNA cloning of gs, gogat, and gdh from Haematococcus pluvialis and transcription and enzyme level analysis in different nitrogen concentration. J Appl Phycol 31(1):183–190
Hu R, Qu FF, Tang JZ, Zhao Q, Yan JP, Zhou ZG, Zhou Y, Liu Z (2017) Cloning, expression, and nutritional regulation of the glutamine synthetase gene in Ctenopharyngodon idellus. Comp Biochem Physiol B:S109649591730074X
Pan LQ, Si LJ, Liu SN, Liu MQ, Wang GH (2018) Levels of metabolic enzymes and nitrogenous compounds in the swimming crab Portunus trituberculatus exposed to elevated ambient ammonia-N. J Ocean Univ China 17(4):957–966
Peng RB, Le KX, Wang PS, Wang Y, Han QX, Jiang XM (2017) Detoxification pathways in response to environmental ammonia exposure of the cuttlefish, Sepia pharaonis: glutamine and urea formation. J World Aquacult Soc 48(2):342–352
Ferretti JA, Calesso DF (2011) Toxicity of ammonia to surf clam (Spisula solidissima) larvae in saltwater and sediment elutriates. Mar Environ Res 71(3):189–194
Wang XQ, Wang LL, Yao C, Qiu LM, Zhang H, Zhi Z (2012) Alternation of immune parameters and cellular energy allocation of Chlamys farreri under ammonia-N exposure and Vibrio anguillarum challenge. Fish Shellfish Immunol 32(5):741–749
Montresor LC, Miranda-Filho KC, Paglia A, Luz DMR, Araújo JM, Silva MJDS, Gerhard L, Martinez CB, Vidigal THDA (2013) Short-term toxicity of ammonia, sodium hydroxide and a commercial biocide to golden mussel Limnoperna fortunei (Dunker, 1857). Ecotoxicol Environ Saf 92:150–154
Zhao XL, Fu JP, Jiang LT, Zhang WW, Shao YN, Jin CH, Xiong JB, Li CH (2018) Transcriptome-based identification of the optimal reference genes as internal controls for quantitative RT-PCR in razor clam (Sinonovacula constricta). Genes Genomics 40(6):603–613
Willett CS, Burton RS (2003) Characterization of the glutamate dehydrogenase gene and its regulation in a euryhaline copepod. Comp Biochem Physiol B 135(4):639–646
Chakrapani V, Rasal KD, Mohapatra SD, Rasal AR, Jayasankar P, Barman HK (2017) Molecular characterization, computational analysis and transcript profiling of glutamate dehydrogenase (gdh) gene of Macrobrachium rosenbergii exposed to saline water. Gene Rep:S2452014417300377
Liu HY, Sun WW, Tan BP, Chi SY, Dong XH, Yang QH (2012) Molecular cloning and expression of hepatopancreas glutamine synthetase in the Pacific white shrimp, Litopenaeus vannamei, induced by acute hypo-osmotic stress. Aquaculture 362–363(Complete):80–87
Lu ZJ, Qin ZD, Babu VS, Ye CK, Su GM, Li JB, Yang HY, Pan G, Lin L (2019) Expression and functional characterization of glutamine synthetase from giant freshwater prawn (Macrobrachium rosenbergii) under osmotic stress. Aquac Res 2635
Bao YB, Li L, Ye MX, Dong YH, Jin WX, Lin ZH (2013) Expression of glutamine synthetase in Tegillarca granosa (Bivalvia, Arcidae) hemocytes stimulated by Vibrio parahaemolyticus and lipopolysaccharides. Genet Mol Res 12(2):1143–1154
Geng ZX, Liu Q, Wang T, Ma S, Shan HW (2020) Changes in physiological parameters involved in glutamine and urea synthesis in Pacific white shrimp, Litopenaeus vannamei, fed Ampithoe sp. meal and exposed to ammonia stress. Aquac Res 51(7):1–10
Dong XX, Liu QG, Kan DQ, Zhao WH, Guo HS, Lv LL (2020) Effects of ammonia-N exposure on the growth, metabolizing enzymes, and metabolome of Macrobrachium rosenbergii. Ecotoxicol Environ Saf 189:110046
Tang XB, Fu Y, Zhao YR, Pi J, Wang HQ (2020) Dietary α-Ketoglutarate supplementation alleviates harmful effects of high environmental ammonia on grass carp, Ctenopharyngodon idella. Aquac Res 51:1182–1189
Hong ML, Chen LQ, Sun XJ, Gu SZ, Zhang L, Chen Y (2007) Metabolic and immune responses in Chinese mitten-handed crab (Eriocheir sinensis) juveniles exposed to elevated ambient ammonia. Comp Biochem Physiol C 145(3):363–369
Peh WYX, Chew SF, Ching BY, Loong AM, Ip YK (2010) Roles of intestinal glutamate dehydrogenase and glutamine synthetase in environmental ammonia detoxification in the euryhaline four-eyed sleeper, Bostrychus sinensis. Aquat Toxicol 98(1):91–98
Nissen JD, Lykke K, Bryk J, Stridh MH, Zaganas L, Skytt DM, Schousboe A, Bak LK, Enard W, Pääbo S, Waagepetersen HS (2017) Expression of the human isoform of glutamate dehydrogenase, hGDH2, augments TCA cycle capacity and oxidative metabolism of glutamate during glucose deprivation in astrocytes. Glia 65(3):474–488
Zhang X, Pan LQ, Wei C, Tong RX, Li YF, Ding M, Wang HD (2020) Crustacean hyperglycemic hormone (CHH) regulates the ammonia excretion and metabolism in white shrimp, Litopenaeus vannamei under ammonia-N stress. Sci Total Environ 723:138128
Acknowledgements
This work was supported by National Key Research and Development Program of China (2018YFD0901405), Zhejiang Major Program of Science and Technology (2016C02055-9), Ningbo Major Project of Science and Technology (2019B10005), Modern Agro-industry Technology Research System (CARS-49) and Ningbo Top Discipline of Environmental Science and Engineering.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The S. constricta used in this work were collected from the genetic breeding research center of Zhejiang Wanli University, China. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Zhejiang Wanli University, China.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, H., Sun, G., Lin, Z. et al. The razor clam Sinonovacula constricta uses the strategy of conversion of toxic ammonia to glutamine in response to high environmental ammonia exposure. Mol Biol Rep 47, 9579–9593 (2020). https://doi.org/10.1007/s11033-020-06018-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06018-w