Abstract
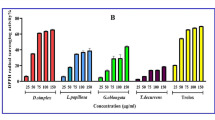
Plant derived products are widely used in cancer treatment. Gall species has been preferred for treatment various diseases in folk medicine, but there are few studies on the anticancer effects of gall species. The present study reports the antioxidant activity and total secondary metabolites of extracts of A. tomentosus galls. The antioxidant potency of galls was carried out using different in-vitro model systems. Their cytotoxic efficacy on Mia-Paca cell line was also explored. Gall extract was found to contain a large amount of phenolic acids. The extract potently scavenged free radicals including DPPH (IC50 9.56 ± 1.08 µg/mL), ABTS (IC50 18.51 ± 0.25 µg/mL). It can be seen as a potential source of antioxidants according to β-carotene/linoleic acid method (%92.58 ± 0.92) and Phosphomolybdenum assays (104.36 ± 4.95 mgAE/g). Gall extract also posses ability of metal chelating (%40.07 ± 2.30) and Fe3+ (184.01 ± 2.83 mgTE/g) and Cu2+ (89.81 ± 0.96 mgTE/g) reducing activity. According to total secondary metabolites tests, gall extract showed high total phenolic, total flavonoid and total tannin amount. HPLC analysis of the extract suggested it to contain caffeic acid (424.068.479 µg/g), ellagic acid (187.696.132 µg/g). XTT assay revealed gall extract to enhance percent survival of Mia-Paca2 cell line exposed A. tomentosus extracts. The best cytotoxic effect was determined in acetone extracts (IC50: 124.7 µM). Expression of some genes (Bax, Bcl-2, FAS, BID, caspase-3, caspase-8, caspase-9, caspase-10, FADD, TRADD) in the apoptosis pathway was determined to invastigate apoptosis inducing activity. These results indicate that A. tomentosus galls possess potent antioxidant activity, when tested both in chemical as well as biological models.
Similar content being viewed by others
References
Ugur D, Gunes H, Gunes F, Mammadov R (2017) Cytotoxic activities of certain medicinal plants on different cancer. Iran J Pharm Res 13:299
Tokgun O, Akca H, Mammadov R, Aykurt C, Deniz G (2012) Convolvulus galaticus, Crocus antalyensis, and Lilium candidum extracts show their antitumor activity through induction of p53-mediated apoptosis on human breast cancer cell line MCF-7 cells. J Med Food 15(11):1000–1005. https://doi.org/10.1089/jmf.2012.0050
Rezzoug M, Bakchiche B, Gherib A, Roberta A, Kilinçarslan Ö, Mammadov R, Bardaweel SK (2019) Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement Altern Med 19(1):146. https://doi.org/10.1186/s12906-019-2556-y
Elmas L, Secme M, Mammadov R, Fahrioglu U, Dodurga Y (2019) The determination of the potential anticancer effects of Coriandrum sativum in PC-3 and LNCaP prostate cancer cell lines. J Cell Biochem 120(3):3506–3513. https://doi.org/10.1002/jcb.27625
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. https://doi.org/10.1002/ijc.29210
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Han Y, Ma L, Zhao L, Feng W, Zheng X (2019) Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed Pharmacother 115:108878. https://doi.org/10.1016/j.biopha.2019.108878
Bhuyan DJ, Vuong QV, Chalmers AC, Bowyer MC, Scarlett CJ (2018) An array of bioactive compounds from Australian eucalypts and their relevance in pancreatic cancer therapeutics. Pancreas 47(6):690–707. https://doi.org/10.1097/MPA.0000000000001074
Chung KT, Wong TY, Wei CI, Huang YW, Lin Y (1998) Tannins and human health: a review. Crit Rev Food Sci Nutr 38:421–464. https://doi.org/10.1080/10408699891274273
Cai Y, Zhang J, Chen NG, Shi Z, Qiu J, He C, Chen M (2017) Recent advances in anticancer activities and drug delivery systems of Tannins. Med Res Rev 37:665–701. https://doi.org/10.1002/med.21422
Chauhan SS, Shetty AB, Hatami E, Chowdhury P, Yallapu MM (2020) Pectin-tannic acid nano-complexes promote the delivery and bioactivity of drugs in pancreatic cancer cells. Pharmaceutics 12(3):285. https://doi.org/10.3390/pharmaceutics12030285
Türkan F, Taslimi P, Saltan FZ (2019) Tannic acid as a natural antioxidant compound: discovery of a potent metabolic enzyme inhibitor for a new therapeutic approach in diabetes and Alzheimer’s disease. J Biochem Mol Toxicol 33(8):e22340. https://doi.org/10.1002/jbt.22340
Claus EP (1962) Pharmacognosy. Acad Med 37(1):79
Sukor N, Jusoh R, Rahim SA, Kamarudin N (2018) Ultrasound assisted methods for enhanced extraction of phenolic acids from Quercus infectoria galls. Mater Today 5(10):21990–21999. https://doi.org/10.1016/j.matpr.2018.07.060
Azmaz M, Kılınçarslan Aksoy Ö, Katılmış Y, Mammadov M (2020) Investigation of the antioxidant activity and phenolic compounds of Andricus quercustozae gall and host plant (Quercus infectoria). Int J Secondary Metab 7(2):77–87. https://doi.org/10.21448/ijsm.674930
Akbar S (2020) Quercus infectoria G. Olivier (Fagaceae). In: Handbook of 200 medicinal plants. pp. 1505–1511. https://doi.org/10.1007/978-3-030-16807-0_155
Tavakoli M, Melika G, Sadeghi SE, Pénzes Z, Assareh MA, Atkinson R et al (2008) New species of oak gallwasps from Iran (Hymenoptera: Cynipidae: Cynipini). Zootaxa 1699(1):1–64
Azmaz M, Katılmış Y (2017) Cynipidae (Insecta: Hymenoptera) fauna of Istanbul. Mun Ent Zool 12(1):151
Ionescu MA (1957) Insecta: Cynipinae. Editura Academiei Republicii Populare România
Melika G (2006) Gall wasps of Ukraine. Cynipidae Vestnik zoologii 21(1–2):301–644
Kılınçarslan O, Mammadov R (2018) HPLC analysis and antioxidant, antibacterial and cytotoxicity activities of various solvent extracts of Erysimum kotschyanum Gay.(Brassicaceae). J Chem Soc Pak 40(04):707
Amin I, Zamaliah MM, Chin WF (2004) Total antioxidant activity and phenolic content in selected vegetables. Food Chem 87:581–586. https://doi.org/10.1016/j.foodchem.2004.01.010
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(29):337–41
Wu C, Chen F, Wang X, Kim HJ, He GQ, Haley-Zitlin V, Huang G (2006) Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem 96(2):220–227. https://doi.org/10.1016/j.foodchem.2005.02.024
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Apak R, Guclu K, Ozyurek M, Karademir SE, Ercag E (2006) The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr 57:292–304. https://doi.org/10.1080/09637480600798132
Apak R, Özyürek M, Güçlü K, Çapanoğlu E (2016) Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Agric Food Chem 64(5):997–1027. https://doi.org/10.1021/acs.jafc.5b04739
Dinis TCP, Madeira VMC, Almeida LM (1994) Action of phenolic derivates (acetoaminophen, salicylate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169. https://doi.org/10.1006/abbi.1994.1485
Slinkard K, Vernon L, Singleton VL (1977) Total phenol analyses: automation and comparison with manual methods. Am J Enol Viticult 28:49–55
Arvouet-Grand A, Vennat B, Pourrat A, Legret P (1994) Standardization of propolis extract and identification of principal constituents. J Pharm Belg 49:462–468
Bekir J, Mars M, Souchard JP, Bouajila J (2013) Assessment of antioxidant, anti inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem Toxicol 55:470–475. https://doi.org/10.1016/j.fct.2013.01.036
Caponio F, Alloggio V, Gomesb T (1999) Phenolic compounds of virgin olive oil; influence of paste preparation techniques. Food Chem 64:203–209. https://doi.org/10.1016/S0308-8146(98)00146-0
Fahrioğlu U, Dodurga Y, Elmas L, Seçme M (2016) Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene 576(1):476–482. https://doi.org/10.1016/j.gene.2015.10.061
Ahmad W (2016) Ethnopharmacology of Quercus infectoria olivier galls: a review. Hippocratic J Unani Med 11:105–18
Shrestha S, Kaushik VS, Eshwarappa RS, Subaramaihha SR, Ramanna LM, Lakkappa DB et al (2014) Pharmacognostic studies of insect gall of Quercus infectoria Olivier (Fagaceae). Asian Pac J Trop Biomed 4(1):35–39. https://doi.org/10.1016/S2221-1691(14)60205-7
Zachariah SM, Kumar NM, Darsana K, Gopal D, Thomas N, Ramkumar M et al (2014) Phytochemical screening, formulation and evaluation of dried galls of Quercus infectoria Olivier. Int J Pharm Sci Rev Res 26:125–30
Fathabada AE, Schariatifar N, Mardania K, Pourfard MI (2015) Study on antibacterial and antioxidant activiy of Oak gall Quercus infectoria (extracts from Iran). Int J Curr Scı 14:44–50
Arina MI, Harisun Y (2019) Effect of extraction temperatures on tannin content and antioxidant activity of Quercus infectoria (Manjakani). Biocatal Agric Biotechnol 19:101104. https://doi.org/10.1016/j.bcab.2019.101104
Kaur G, Athar M, Alam MS (2008) Quercus infectoria galls possess antioxidant activity and abrogates oxidative stress-induced functional alterations in murine macrophages. Chem Biol Interact 171:272–282. https://doi.org/10.1016/j.cbi.2007.10.002
Doğan Abdioğlu M (2019) Bazı meşe gallerinin kolinesteraz, tirozinaz ve üreaz enzim inhibisyonu ile antioksidan aktivitesinin belirlenmesi. Master’s thesis, Batman Üniversitesi Fen Bilimleri Enstitüsü, Türkiye
Shahin S, Ahmad N (2014) Antioxidant properties and total phenolic content of herbs used in post partum diet therapy in Patna (Bihar), India. J Pharm Biol Sci 9:17–20
Sukor NF, Jusoh R, Kamarudin NS, Halim NA, Sulaiman AZ, Abdullah SB (2020) Synergistic effect of probe sonication and ionic liquid for extraction of phenolic acids from oak galls. Ultrason Sonochem 62:104876. https://doi.org/10.1016/j.ultsonch.2019.104876
Aydin Ç, Rakhimzhanova A, Kilinçarslan Ö, Mammadov R (2020) Antioxidant and phenolic characterization with HPLC of various extract of Verbascum glomeratum Linneus. J Chem Soc Pak 42(2):222
Hasmah A, Nurazila Z, Chow CY, Rina R, Rafiquzzaman M (2010) Cytotoxic effects of Quercus infectoria extracts towards cervical (Hela) and Ovarian (Caov-3) cancer cell lines. Health Environ J 1(2):17–23
Gao J, Yang X, Yin W, Li M (2018) Gallnuts: a potential treasure in anticancer drug discovery. Evid Based Complement Altern Med 2018:4930371
Jalill RDA (2018) Chemical analysis and anticancer effects of Juniperus polycarpos and oak gall plants extracts. Res J Pharm Technol 11(6):2372–2387
Abdalan S, Baghbani-Arani F, Sadat Shandiz SA (2018) Evaluation of anticancer effect of aqueous and hydroalcoholic extracts of Quercusin fectoria leaf against colon cancer HT29 cell line. J Arak Univ Med Sci 21(4):48–57
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med 49(11):1603–1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
Acknowledgements
The authors are grateful for the financial support from the Pamukkale University Scientific Research Projects Coordination Unit (Project No: 2018FEBE062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kılınçarslan Aksoy, Ö., Mammadov, R. & Seçme, M. Antioxidant activity, phytochemical composition of Andricus tomentosus and its antiproliferative effect on Mia-Paca2 cell line. Mol Biol Rep 47, 7633–7641 (2020). https://doi.org/10.1007/s11033-020-05833-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05833-5