Abstract
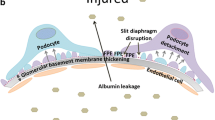
Diabetic kidney disease (DKD) is an important diabetic microvascular complication, which has become the main cause of end-stage renal disease (ESRD) all over the world. It is of great significance to find effective therapeutic targets and improve the prognosis of the disease. Traditionally, it is believed that the activation of the renin–angiotensin–aldosterone system (RAAS) is the main reason for the progression of DKD, but with the progress of research, it is known that the production of proteinuria in patients with DKD is also related to podocyte injury and loss. Many studies have shown that mitochondrial dysfunction in podocytes plays an important role in the occurrence and development of DKD, and oxidative stress is also the main pathway and common hub of diabetes to the occurrence and development of microvascular and macrovascular complications. Thus, the occurrence and progression of DKD is correlated with not only the activation of the RAAS, but also the damage of mitochondria, oxidative stress, and inflammatory mediators. Besides, diabetes-related metabolic disorders can also cause abnormalities in mitochondrial dynamics, autophagy and cellular signal transduction, which are intertwined in a complex way. Therefore, in this review, we mainly explore the mechanism and the latest research progress of podocyte mitochondria in DKD and summarize the main signal pathways involved in them. Thus, it provides feasible clinical application and future research suggestions for the prevention and treatment of DKD, which has important practical significance for the later treatment of patients with DKD.



Similar content being viewed by others
References
Lindblom R, Higgins G, Coughlan M, de Haan JB (2015) Targeting mitochondria and reactive oxygen species-driven pathogenesis in diabetic nephropathy. Rev Diabet Stud 12(1–2):134–156. https://doi.org/10.1900/RDS.2015.12.134
Schiffer TA, Friederich-Persson M (2017) Mitochondrial reactive oxygen species and kidney hypoxia in the development of diabetic nephropathy. Front Physiol 8:211. https://doi.org/10.3389/fphys.2017.00211
Lewis KUJB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71(6):884–895. https://doi.org/10.1053/j.ajkd.2017.10.026
Sagoo MK, Gnudi L (2018) Diabetic nephropathy: is there a role for oxidative stress? Free Radic Biol Med 116:50–63. https://doi.org/10.1016/j.freeradbiomed.2017.12.040
Ling-Feng Z, Xiao Y, Sun L (2019) A glimpse of the mechanisms related to renal fibrosis in diabetic nephropathy. Adv Exp Med Biol 1165:49–79
Bose M, Almas S, Prabhakar S (2017) Wnt signaling and podocyte dysfunction in diabetic nephropathy. J Investig Med 65(8):1093–1101. https://doi.org/10.1136/jim-2017-000456
Imasawa T, Obre E, Bellance N, Lavie J, Imasawa T, Rigothier C et al (2017) High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. FASEB J 31(1):294–307. https://doi.org/10.1096/fj.201600293R
Dai H, Liu Q, Liu B (2017) Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res 2017:1–10. https://doi.org/10.1155/2017/2615286
Rodewald R, Karnovsky MJ (1974) Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60(2):423–433
Grahammer F, Wigge C, Schell C, Kretz O, Patrakka J, Schneider S et al (2016) A flexible, multilayered protein scaffold maintains the slit in between glomerular podocytes. JCI Insight. https://doi.org/10.1172/jci.insight.86177
Nagata M (2016) Podocyte injury and its consequences. Kidney Int 89(6):1221–1230. https://doi.org/10.1016/j.kint.2016.01.012
Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z (2015) Mitochondrial function and disturbances in the septic kidney. Semin Nephrol 35(1):108–119. https://doi.org/10.1016/j.semnephrol.2015.01.011
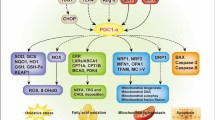
Sharma K (2017) Mitochondrial dysfunction in the diabetic kidney. Adv Exp Med Biol 982:553–562. https://doi.org/10.1007/978-3-319-55330-6_28
Lee SY, Choi ME (2015) Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol 30(7):1063–1075. https://doi.org/10.1007/s00467-014-2888-2
Liu M, Liang K, Zhen J, Zhou M, Wang X, Wang Z et al (2017) Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 8(1):413. https://doi.org/10.1038/s41467-017-00498-4
Tung CW, Hsu YC, Shih YH, Chang PJ, Lin CL (2018) Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology (Carlton, Vic) 23(Suppl 4):32–37. https://doi.org/10.1111/nep.13451
Coward R, Fornoni A (2015) Insulin signaling: implications for podocyte biology in diabetic kidney disease. Curr Opin Nephrol Hypertens 24(1):104–110. https://doi.org/10.1097/MNH.0000000000000078
Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S et al (2015) Diabetic kidney disease. Nat Rev Dis Primers 1:15018. https://doi.org/10.1038/nrdp.2015.18
Lin F, Bao Y-W, Wu F-G (2018) Improving the phototherapeutic efficiencies of molecular and nanoscale materials by targeting mitochondria. Molecules. https://doi.org/10.3390/molecules23113016
Flemming NB, Gallo LA, Forbes JM (2018) Mitochondrial dysfunction and signaling in diabetic kidney disease: oxidative stress and beyond. Semin Nephrol 38(2):101–110. https://doi.org/10.1016/j.semnephrol.2018.01.001
Forbes JM, Thorburn DR (2018) Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol 14(5):291–312. https://doi.org/10.1038/nrneph.2018.9
Hamanaka RB, Chandel NS (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35(9):505–513. https://doi.org/10.1016/j.tibs.2010.04.002
Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL et al (1987) Oxygen radicals and human disease. Ann Intern Med 107(4):526–545
Rhee SG (2006) Cell signaling. H2O2, a necessary evil for cell signaling. Science 312(5782):1882–1883
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J. https://doi.org/10.1042/BJ20081386
Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD (2013) Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol 1:304–312. https://doi.org/10.1016/j.redox.2013.04.005
Nickel A, Kohlhaas M, Maack C (2014) Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol 73:26–33. https://doi.org/10.1016/j.yjmcc.2014.03.011
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol (Lond) 552(Pt 2):335–344
Fridovich I (1997) Superoxide anion radical (O2−·), superoxide dismutases, and related matters. J Biol Chem 272(30):18515–18517
Holmström KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15(6):411–421. https://doi.org/10.1038/nrm3801
Chandel NS (2015) Evolution of mitochondria as signaling organelles. Cell Metab 22(2):204–206. https://doi.org/10.1016/j.cmet.2015.05.013
Yang S, Han Y, Liu J, Song P, Xu X, Zhao L et al (2017) Mitochondria: a novel therapeutic target in diabetic nephropathy. Curr Med Chem 24(29):3185–3202. https://doi.org/10.2174/0929867324666170509121003
Jha JC, Banal C, Chow BSM, Cooper ME, Jandeleit-Dahm K (2016) Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal 25(12):657–684
Nishikawa T, Brownlee M, Araki E (2015) Mitochondrial reactive oxygen species in the pathogenesis of early diabetic nephropathy. J Diabetes Investig 6(2):137–139. https://doi.org/10.1111/jdi.12258
Jha JC, Ho F, Dan C, Jandeleit-Dahm K (2018) A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci (Lond, Engl, 1979). 132(16):1811–1836. https://doi.org/10.1042/cs20171459
Bargiela D, Burr SP, Chinnery PF (2018) Mitochondria and hypoxia: metabolic crosstalk in cell-fate decisions. Trends Endocrinol Metab 29(4):249–259. https://doi.org/10.1016/j.tem.2018.02.002
Herr CQ, Hausinger RP (2018) Amazing diversity in biochemical roles of Fe(II)/2-oxoglutarate oxygenases. Trends Biochem Sci 43(7):517–532. https://doi.org/10.1016/j.tibs.2018.04.002
Fuhrmann DC, Brune B (2017) Mitochondrial composition and function under the control of hypoxia. Redox Biol 12:208–215. https://doi.org/10.1016/j.redox.2017.02.012
Thomas JL, Pham H, Li Y, Hall E, Perkins GA, Ali SS et al (2017) Hypoxia-inducible factor-1α activation improves renal oxygenation and mitochondrial function in early chronic kidney disease. Am J Physiol Renal Physiol 313(2):F282–F290. https://doi.org/10.1152/ajprenal.00579.2016
Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M et al (2015) Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol JASN 26(2):328–338. https://doi.org/10.1681/ASN.2013090990
Persson P, Palm F (2017) Hypoxia-inducible factor activation in diabetic kidney disease. Curr Opin Nephrol Hypertens 26(5):345–350. https://doi.org/10.1097/MNH.0000000000000341
Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132. https://doi.org/10.1146/annurev-cellbio-092910-154005
Wang P, Mugume Y, Bassham DC (2018) New advances in autophagy in plants: regulation, selectivity and function. Semin Cell Dev Biol 80:113–122. https://doi.org/10.1016/j.semcdb.2017.07.018
Randow F, Youle RJ (2014) Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe 15(4):403–411. https://doi.org/10.1016/j.chom.2014.03.012
Maejima I, Takahashi A, Omori H, Kimura T, Takabatake Y, Saitoh T et al (2013) Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J 32(17):2336–2347. https://doi.org/10.1038/emboj.2013.171
Duan WJ, Li YF, Liu FL, Deng J, Wu YP, Yuan WL et al (2016) A SIRT3/AMPK/autophagy network orchestrates the protective effects of trans-resveratrol in stressed peritoneal macrophages and RAW 264.7 macrophages. Free Radic Biol Med 95:230–242. https://doi.org/10.1016/j.freeradbiomed.2016.03.022
Roy S, Kim D, Sankaramoorthy A (2019) Mitochondrial structural changes in the pathogenesis of diabetic retinopathy. J Clin Med. https://doi.org/10.3390/jcm8091363
Zhao Y, Guo Y, Jiang Y, Zhu X, Liu Y, Zhang X (2017) Mitophagy regulates macrophage phenotype in diabetic nephropathy rats. Biochem Biophys Res Commun 494(1–2):42–50
Ding Y, Choi ME (2015) Autophagy in diabetic nephropathy. J Endocrinol 224(1):R15–R30. https://doi.org/10.1530/JOE-14-0437
Smith MA, Covington MD, Schnellmann RG (2012) Loss of calpain 10 causes mitochondrial dysfunction during chronic hyperglycemia. Arch Biochem Biophys 523(2):161–168. https://doi.org/10.1016/j.abb.2012.04.020
Zhan M, Usman IM, Sun L, Kanwar YS (2015) Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol JASN 26(6):1304–1321. https://doi.org/10.1681/ASN.2014050457
Higgins GC, Coughlan MT (2014) Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol 171(8):1917–1942. https://doi.org/10.1111/bph.12503
Long J, Badal SS, Ye Z, Wang Y, Ayanga BA, Galvan DL et al (2016) Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Investig 126(11):4205–4218. https://doi.org/10.1172/JCI87927
Kasashima K, Sumitani M, Satoh M, Endo H (2008) Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp Cell Res 314(5):988–996. https://doi.org/10.1016/j.yexcr.2008.01.005
Merkwirth C, Martinelli P, Korwitz A, Morbin M, Brönneke HS, Jordan SD et al (2012) Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet 8(11):e1003021. https://doi.org/10.1371/journal.pgen.1003021
Ising C, Koehler S, Brähler S, Merkwirth C, Höhne M, Baris OR et al (2015) Inhibition of insulin/IGF-1 receptor signaling protects from mitochondria-mediated kidney failure. EMBO Mol Med 7(3):275–287. https://doi.org/10.15252/emmm.201404916
Mallipattu SK, Horne SJ, D'Agati V, Narla G, Liu R, Frohman MA et al (2015) Krüppel-like factor 6 regulates mitochondrial function in the kidney. J Clin Investig 125(3):1347–1361. https://doi.org/10.1172/JCI77084
Horne SJ, Vasquez JM, Guo Y, Ly V, Piret SE, Leonardo AR et al (2018) Podocyte-specific loss of Kruppel-like factor 6 increases mitochondrial injury in diabetic kidney disease. Diabetes 67(11):2420–2433. https://doi.org/10.2337/db17-0958
Ducasa GM, Mitrofanova A, Mallela SK, Liu X, Molina J, Sloan A et al (2019) ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J Clin Invest 129(8):3387–3400. https://doi.org/10.1172/JCI125316
Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U (2014) Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 55(3):561–572. https://doi.org/10.1194/jlr.P040501
Wang N, Silver DL, Thiele C, Tall AR (2001) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 276(26):23742–23747
Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G (2014) Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837(4):408–417. https://doi.org/10.1016/j.bbabio.2013.10.006
Kawanami D, Matoba K, Utsunomiya K (2016) Signaling pathways in diabetic nephropathy. Histol Histopathol 31(10):1059–1067. https://doi.org/10.14670/hh-11-777
Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H et al (2007) Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6(1):55–68
Szrejder M, Piwkowska A (2019) AMPK signalling: implications for podocyte biology in diabetic nephropathy. Biol Cell 111(5):109–120. https://doi.org/10.1111/boc.201800077
Hardie DG, Scott JW, Pan DA, Hudson ER (2003) Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546(1):113–120
Kurumbail RG, Calabrese MF (2012) Structure and regulation of AMPK. Exp Suppl 2016:107
Szymańska P, Martin KR, MacKeigan JP, Hlavacek WS, Lipniacki T (2015) Computational analysis of an autophagy/translation switch based on mutual inhibition of MTORC1 and ULK1. PLoS ONE 10(3):e0116550. https://doi.org/10.1371/journal.pone.0116550
Willows R, Sanders MJ, Xiao B, Patel BR, Martin SR, Read J et al (2017) Phosphorylation of AMPK by upstream kinases is required for activity in mammalian cells. Biochem J 474(17):3059–3073. https://doi.org/10.1042/bcj20170458
Nishi H, Higashihara T, Inagi R (2019) Lipotoxicity in kidney, heart, and skeletal muscle dysfunction. Nutrients. https://doi.org/10.3390/nu11071664
Kim Y, Park CW (2016) Adenosine monophosphate-activated protein kinase in diabetic nephropathy. Kidney Res Clin Pract 35(2):69–77. https://doi.org/10.1016/j.krcp.2016.02.004
Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ et al (2018) The adiponectin receptor agonist AdipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol JASN 29(4):1108–1127. https://doi.org/10.1681/asn.2017060627
Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D (2007) Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem 282(45):32539–32548
Nusse R, Clevers H (2017) Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169(6):985–999
Zhou D, Tan RJ, Fu H, Liu Y (2016) Wnt/β-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest 96(2):156–167. https://doi.org/10.1038/labinvest.2015.153
Luo C, Zhou S, Zhou Z, Liu Y, Yang L, Liu J et al (2018) Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J Am Soc Nephrol JASN 29(4):1238–1256. https://doi.org/10.1681/ASN.2017050574
Wang Y, Zhou CJ, Liu Y (2018) Wnt Signaling in kidney development and disease. Prog Mol Biol Transl Sci 153:181–207
Feng Y, Ren J, Gui Y, Wei W, Shu B, Lu Q et al (2018) Wnt/-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol JASN 29(1):182–193. https://doi.org/10.1681/ASN.2017040391
Miao J, Liu J, Niu J, Zhang Y, Shen W, Luo C et al (2019) Wnt/beta-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 18(5):e13004. https://doi.org/10.1111/acel.13004
Liu B-L, Chen Y-P, Cheng H, Wang Y-Y, Rui H-L, Yang M et al (2015) The protective effects of curcumin on obesity-related glomerulopathy are associated with inhibition of Wnt/β-catenin signaling activation in podocytes. Evid Based Complement Altern Med 2015:827472. https://doi.org/10.1155/2015/827472
Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM et al (2011) Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem 286(29):26003–26015. https://doi.org/10.1074/jbc.M111.223164
Zhang H, Mao X, Sun Y, Hu R, Luo W, Zhao Z et al (2015) NF-κB upregulates ubiquitin C-terminal hydrolase 1 in diseased podocytes in glomerulonephritis. Mol Med Rep 12(2):2893–2901. https://doi.org/10.3892/mmr.2015.3780
Zhang H, Luo W, Sun Y, Qiao Y, Zhang L, Zhao Z et al (2016) Wnt/β-catenin signaling mediated-UCH-L1 expression in podocytes of diabetic nephropathy. Int J Mol Sci. https://doi.org/10.3390/ijms17091404
Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ (2010) Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev 24(14):1507–1518. https://doi.org/10.1101/gad.1924910
Li Z, Xu J, Xu P, Liu S, Yang Z (2013) Wnt/β-catenin signalling pathway mediates high glucose induced cell injury through activation of TRPC6 in podocytes. Cell Prolif 46(1):76–85. https://doi.org/10.1111/cpr.12010
Saxton RA, Sabatini DM (2017) mTOR Signaling in growth, metabolism, and disease. Cell 169(2):361–371
Kajiwara M, Masuda S (2016) Role of mTOR inhibitors in kidney disease. Int J Mol Sci. https://doi.org/10.3390/ijms17060975
Yao Y, Wang J, Yoshida S, Nada S, Okada M, Inoki K (2016) Role of regulator in the regulation of mechanistic target of rapamycin signaling in podocytes and glomerular function. J Am Soc Nephrol JASN 27(12):3653–3665
Skała E, Sitarek P, Toma M, Szemraj J, Radek M, Nieborowska-Skorska M et al (2016) Inhibition of human glioma cell proliferation by altered Bax/Bcl-2-p53 expression and apoptosis induction by Rhaponticum carthamoides extracts from transformed and normal roots. J Pharm Pharmacol 68(11):1454–1464. https://doi.org/10.1111/jphp.12619
Shi H, Zhang A, He Y, Yang M, Gan W (2016) Effects of p53 on aldosterone-induced mesangial cell apoptosis in vivo and in vitro. Mol Med Rep 13(6):5102–5108. https://doi.org/10.3892/mmr.2016.5156
Morita M, Gravel S-P, Chénard V, Sikström K, Zheng L, Alain T et al (2013) mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab 18(5):698–711. https://doi.org/10.1016/j.cmet.2013.10.001
Fantus D, Rogers NM, Grahammer F, Huber TB, Thomson AW (2016) Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat Rev Nephrol 12(10):587–609. https://doi.org/10.1038/nrneph.2016.108
Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J et al (2015) mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 14(4):473–480. https://doi.org/10.4161/15384101.2014.991572
Gao P, Li L, Yang L, Gui D, Zhang J, Han J et al (2019) Yin Yang 1 protein ameliorates diabetic nephropathy pathology through transcriptional repression of TGFβ1. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaw2050
Yao Y, Inoki K (2016) The role of mechanistic target of rapamycin in maintenance of glomerular epithelial cells. Curr Opin Nephrol Hypertens 25(1):28–34. https://doi.org/10.1097/MNH.0000000000000181
Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S et al (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Investig 121(6):2181–2196. https://doi.org/10.1172/JCI44771
Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S et al (2011) Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Investig 121(6):2197–2209. https://doi.org/10.1172/JCI44774
Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C et al (2013) AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19(10):1288–1296. https://doi.org/10.1038/nm.3313
Funding
This paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, J., Ye, D., Gao, C. et al. Mechanism of progression of diabetic kidney disease mediated by podocyte mitochondrial injury. Mol Biol Rep 47, 8023–8035 (2020). https://doi.org/10.1007/s11033-020-05749-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05749-0