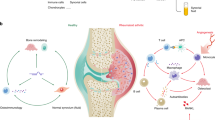
Abstract
Rheumatoid Arthritis (RA) is an autoimmune disease with unknown etiology and a global incidence around 1%, a positive family history increases the risk of RA roughly three to five times. Pain is one of the first symptoms to appear in this disease. MicroRNAs (miRNAs) belong to the class of small non-coding RNAs; they regulate multiple cellular processes including embryonic development, cellular proliferation, differentiation and apoptosis among others. A great deal of evidence points to the employment of miRNAs as therapeutic targets and biomarkers for several pathologies. The main objective of this Review is to assess how miRNAs participate in the pathogenesis of RA. Two advanced searches were conducted in databases, one using “micro-RNA” and “rheumatoid arthritis” as key words, and another one with “micro-RNA”, “pain” and “nociception”. In this Review, we describe how six miRNAs: miR-16-5p, miR-23b-3b, miR-124-3p, miR-146a-5p, miR-155-5p and miR-223-3p, involved in the modulation and transmission of the nociceptive input are unregulated in RA patients. Key molecular pathways involved in nociception, inflammation and autoimmune responses, are regulated by these miRNAs; the NF-κB, TNF-α, interleukins and TLR4. By means of gene repression, the miRNAs here described modulate the nociceptive process as well as the autoimmune response that characterize this disease.

Similar content being viewed by others
References
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388:2023–2038
Ma VY, Chan L, Carruthers KJ (2014) Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pa. Arch Phys Med Rehabil 95:986–995
Bergström M, Ahlstrand I, Thyberg I, et al (2017) ‘Like the worst toothache you’ve had’ – How people with rheumatoid arthritis describe and manage pain. Scand J Occup Ther 1–9.
de Luca K, Parkinson L, Downie A et al (2017) Three subgroups of pain profiles identified in 227 women with arthritis: a latent class analysis. Clin Rheumatol 36:625–634
Björk M, Gerdle B, Thyberg I, Peolsson M (2008) Multivariate relationships between pain intensity and other aspects of health in rheumatoid arthritis–cross sectional and five year longitudinal analyses (the Swedish TIRA project). Disabil Rehabil 30:1429–1438
Guler MA, Celik OF, Ayhan FF (2020) The important role of central sensitization in chronic musculoskeletal pain seen in different rheumatic diseases. Clin Rheumatol 39:269–274. https://doi.org/10.1007/s10067-019-04749-1
Iwakawa H, Tomari Y (2015) The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol 25:651–665
Ryan B, Joilin G, Williams JM (2015) Plasticity-related microRNA and their potential contribution to the maintenance of long-term potentiation. Front Mol Neurosci 8.
Sun X, Zhang J (2014) Identification of putative pathogenic SNPs implied in schizophrenia-associated miRNAs. BMC Bioinformatics 15:194
Higa GSV, de Sousa E, Walter LT et al (2014) MicroRNAs in neuronal communication. Mol Neurobiol 49:1309–1326
Bai G, Ambalavanar R, Wei D, Dessem D (2007) Downregulation of selective microRNAs in trigeminal ganglion neurons following inflammatory muscle pain. Mol Pain 3:15
Zhao J, Lee M-C, Momin A et al (2010) Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J Neurosci 30:10860–10871
Trenkmann M, Brock M, Ospelt C, Gay S (2010) Epigenetics in rheumatoid arthritis. Clin Rev Allergy Immunol 39:10–19
Ospelt C (2016) Epigenetic biomarkers in rheumatology–the future? Swiss Med Wkly 146:w14312
Salehi E, Eftekhari R, Oraei M et al (2015) MicroRNAs in rheumatoid arthritis. Clin Rheumatol 34:615–628
Stanczyk J, Pedrioli DML, Brentano F et al (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheumatol 58:1001–1009
Liu C, Pan A, Chen X et al (2019) MiR-5571-3p and miR-135b-5p, derived from analyses of microRNA profile sequencing, correlate with increased disease risk and activity of rheumatoid arthritis. Clin Rheumatol 38:1753–1765
Niederberger E, Kynast K, Lötsch J, Geisslinger G (2011) MicroRNAs as new players in the pain game. Pain 152:1455–1458
Nakanishi K, Nakasa T, Tanaka N et al (2010) Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal Cord 48:192
Taj SH, Kho W, Riou A et al (2016) MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 91:151–165
de la Rica L, Urquiza JM, Gómez-Cabrero D et al (2013) Identification of novel markers in rheumatoid arthritis through integrated analysis of DNA methylation and microRNA expression. J Autoimmun 41:6–16
Tavasolian F, Abdollahi E, Rezaei R et al (2017) Altered Expression of MicroRNAs in Rheumatoid Arthritis. J Cell Biochem 119(1):478–487
Kynast KL, Russe OQ, Möser C V, et al (2013) Modulation of central nervous system–specific microRNA-124a alters the inflammatory response in the formalin test in mice. PAIN® 154:368–376
Nakamachi Y, Kawano S, Takenokuchi M et al (2009) MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheumatol 60:1294–1304
Willemen HLDM, Huo X-J, Mao-Ying Q-L et al (2012) MicroRNA-124 as a novel treatment for persistent hyperalgesia. J Neuroinflamm 9:143
Li K, Tie H, Hu N et al (2014) Association of two polymorphisms rs2910164 in miRNA-146a and rs3746444 in miRNA-499 with rheumatoid arthritis: a meta-analysis. Hum Immunol 75:602–608
Moszyńska A, Gebert M, Collawn JF, Bartoszewski R (2017) SNPs in microRNA target sites and their potential role in human disease. Open Biol 7:170019
Song Y-J, Li G, He J-H et al (2015) Bioinformatics-based identification of microRNA-regulated and rheumatoid arthritis-associated genes. PLoS ONE 10:e0137551
Lee YH, Bae S-C (2015) The miR-146a polymorphism and susceptibility to systemic lupus erythematosus and rheumatoid arthritismiR-146a-Polymorphismus und Anfälligkeit für systemischen Lupus erythematosus sowie rheumatoide Arthritis. Z Rheumatol 74:153–156
Pauley KM, Satoh M, Chan AL et al (2008) Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther 10:R101
Chen Z-Z, Zhang X-D, Chen Y, Wu Y-B (2017) The role of circulating miR-146a in patients with rheumatoid arthritis treated by Tripterygium wilfordii Hook F. Medicine 96:20(e6775)
Johnnidis JB, Harris MH, Wheeler RT et al (2008) Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451:1125
Pohl K, Yeol J, Ongl W (2011) MicroRNA changes in the mouse prefrontal cortex after inflammatory pain. Eur J Pain 15:801 e1–801. e12
Bas DB, Su J, Wigerblad G, Svensson CI (2016) Pain in rheumatoid arthritis: models and mechanisms. Pain Manag 6:265–284
Lu Y, Cao D-L, Jiang B-C et al (2015) MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav Immun 49:119–129
Alsaleh G, Suffert G, Semaan N et al (2009) Bruton’s tyrosine kinase is involved in miR-346-related regulation of IL-18 release by lipopolysaccharide-activated rheumatoid fibroblast-like synoviocytes. J Immunol 182:5088–5097
Chan EKL, Ceribelli A, Satoh M (2013) MicroRNA-146a in autoimmunity and innate immune responses. Ann Rheum Dis 72:ii90–ii95
Hörber S, Hildebrand DG, Lieb WS et al (2016) The atypical inhibitor of NF-κB, IκBζ, controls macrophage interleukin-10 expression. J Biol Chem Jbc M116:718825
Lindenblatt C, Schulze-Osthoff K, Totzke G (2009) IκBζ expression is regulated by miR-124a. Cell Cycle 8:2019–2023
Lv F, Huang Y, Lv W et al (2017) MicroRNA-146a ameliorates inflammation via TRAF6/NF-κB pathway in intervertebral disc cells. Med Sci Monit Int Med J Exp Clin Res 23:659
Harraz MM, Xu J-C, Guiberson N et al (2014) MiR-223 regulates the differentiation of immature neurons. Mol Cell Ther 2:18
Pandis I, Ospelt C, Karagianni N, et al (2012) Identification of microRNA-221/222 and microRNA-323–3p association with rheumatoid arthritis via predictions using the human tumour necrosis factor transgenic mouse model. Ann Rheum Dis annrheumdis-2011–200803
Fuller-Carter PI, Carter KW, Anderson D et al (2015) Integrated analyses of zebrafish miRNA and mRNA expression profiles identify miR-29b and miR-223 as potential regulators of optic nerve regeneration. BMC Genomics 16:591
Taganov KD, Boldin MP, Chang K-J, Baltimore D (2006) NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci 103:12481–12486
Firestein GS (2004) NF-κB: Holy grail for rheumatoid arthritis? Arthritis Rheum 50:2381–2386
Tian T, Zhou Y, Feng X et al (2016) MicroRNA-16 is putatively involved in the NF-κB pathway regulation in ulcerative colitis through adenosine A2a receptor (A2aAR) mRNA targeting. Sci Rep 6:30824
Filková M, Aradi B, Šenolt L et al (2014) Association of circulating miR-223 and miR-16 with disease activity in patients with early rheumatoid arthritis. Ann Rheum Dis 73:1898–1904
Kusuda R, Cadetti F, Ravanelli MI et al (2011) Differential expression of microRNAs in mouse pain models. Mol Pain 7:17
Zhu S, Pan W, Song X et al (2012) The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med 18:1077
Castro-Villegas C, Pérez-Sánchez C, Escudero A et al (2015) Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFα. Arthritis Res Ther 17:49
Tan Y, Yang J, Xiang K et al (2015) Suppression of microRNA-155 attenuates neuropathic pain by regulating SOCS1 signalling pathway. Neurochem Res 40:550–560
Kurowska-Stolarska M, Alivernini S, Ballantine LE et al (2011) MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci 108:11193–11198
Im YB, Jee MK, Choi JI et al (2012) Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis 3:e426
Nakasa T, Miyaki S, Okubo A et al (2008) Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheumatol 58:1284–1292
Mcinnes IB, Schett G (2011) The Pathogenesis of Rheumatoid Arthritis. 2205–2219
Kawano S, Nakamachi Y (2011) miR-124a as a key regulator of proliferation and MCP-1 secretion in synoviocytes from patients with rheumatoid arthritis. Ann Rheum Dis 70:i88–i91
Heyn J, Luchting B, Hinske LC et al (2016) miR-124a and miR-155 enhance differentiation of regulatory T cells in patients with neuropathic pain. J Neuroinflam 13:248
Martens PB, Goronzy JJ, Schaid D, Weyand CM (1997) Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheumatol 40:1106–1114
Sebastiani GD, Fulci V, Niccolini S et al (2011) Over-expression of miR-223 in T-lymphocytes of early rheumatoid arthritis patients. Clin Exp Rheumatol Suppl 29:1058
López-González MJ, Landry M, Favereaux A (2017) MicroRNA and chronic pain: from mechanisms to therapeutic potential. Pharmacol Ther 180:1–15
Niederberger E, Resch E, Parnham MJ, Geisslinger G (2017) Drugging the pain epigenome. Nat Rev Neurol 13(7):434
Moore LB, Sawyer AJ, Saucier-Sawyer J et al (2016) Nanoparticle delivery of miR-223 to attenuate macrophage fusion. Biomaterials 89:127–135
Funding
Not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are not any conflicts of interest regarding this publication.
Consent for publication
All authors approve the publication of the current version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reyes-Long, S., Cortes-Altamirano, J.L., Clavijio-Cornejo, D. et al. Nociceptive related microRNAs and their role in rheumatoid arthritis. Mol Biol Rep 47, 7265–7272 (2020). https://doi.org/10.1007/s11033-020-05700-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05700-3