Abstract
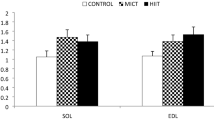
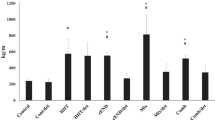
The neuromuscular junction underwent adaptations to meet the demands of muscles following increased muscle activity. This study aimed to investigate the effects of high-intensity interval training (HIIT), endurance training (END), and mixed interval training (MIX) on the gene expression of the calcitonin gene-related peptide-α (CGRP-α), CGRP receptor, nicotinic acetylcholine receptors (nAchR)-β and glial-derived neurotrophic factor (GDNF) among different muscle types. Male Wistar rats were randomly divided into four groups: Control (n = 8), END (n = 8), HIIT (n = 8), and MIX (n = 8). The animals run each training protocol for 8 weeks (five sessions/week). Forty-eight hours after the last training session, the muscles gastrocnemius and soleus were excised under the sterilized situation. After collection, the material was prepared for RNA extraction, Reverse Transcriptase reaction, and qPCR assay. The HIIT training up-regulated the CGRP-α (p < 0.01), CGRP-Rec (p < 0.01), and GDNF (p < 0.01) in soleus as well as the nAchR-β (p < 0.01) and GDNF (p < 0.01) in gastrocnemius muscles. END training down-regulated the gene expression of CGRP-α (p < 0.01), and nAchR-β (p < 0.01) in gastrocnemius but up-regulated nAchR-β (p = 0.037) in soleus and GDNF (p < 0.01) in gastrocnemius muscles. MIX training did not show any significant up or down-regulation. The endurance performance of HIIT and MIX groups was higher than the END group (p < 0.01). All studied genes up-regulated by HIIT training in a muscle type-specific manner. It seems that the improvement of some synaptic indices induced by HIIT resulted in the improvement of endurance performance.
Graphical abstract




Similar content being viewed by others
References
Deschenes MR, Tufts HL, Noronha AL, Li S (2019) Both aging and exercise training alter the rate of recovery of neuromuscular performance of male soleus muscles. Biogerontology 20(2):213–223. https://doi.org/10.1007/s10522-018-9788-y
Krause Neto W, Ciena AP, Anaruma CA, de Souza RR, Gama EF (2015) Effects of exercise on neuromuscular junction components across age: systematic review of animal experimental studies. BMC Res Notes 8(1):713. https://doi.org/10.1186/s13104-015-1644-4
Wilson RJ, Drake JC, Cui D, Ritger ML, Guan Y, Call JA et al (2019) Voluntary running protects against neuromuscular dysfunction following hindlimb ischemia-reperfusion in mice. J Appl Physiol (1985) 126(1):193–201. https://doi.org/10.1152/japplphysiol.00358.2018
Guarino SR, Canciani A, Forneris F (2019) Dissecting the extracellular complexity of neuromuscular junction organizers. Front Mol Biosci 6:156. https://doi.org/10.3389/fmolb.2019.00156
Nishimune H, Stanford JA, Mori Y (2014) Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 49(3):315–324. https://doi.org/10.1002/mus.24095
Csillik B, Tajti L, Kovacs T, Kukla E, Rakic P, Knyihar-Csillik E (1993) Distribution of calcitonin gene-related peptide in vertebrate neuromuscular junctions: relationship to the acetylcholine receptor. J Histochem Cytochem 41(10):1547–1555. https://doi.org/10.1177/41.10.8245413
Popper P, Micevych PE (1989) Localization of calcitonin gene-related peptide and its receptors in a striated muscle. Brain Res 496(1–2):180–186. https://doi.org/10.1016/0006-8993(89)91064-0
Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM (1982) Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 298(5871):240–244. https://doi.org/10.1038/298240a0
Brain S, Williams T, Tippins J, Morris H, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature. 313(5997):54–56. https://doi.org/10.1038/313054a0
Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J et al (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 304(5922):129–135. https://doi.org/10.1038/304129a0
Brain SD, Grant AD (2004) Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84(3):903–934. https://doi.org/10.1152/physrev.00037.2003
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94(4):1099–1142. https://doi.org/10.1152/physrev.00034.2013
Fernandez HL, Ross GS, Nadelhaft I (1999) Neurogenic calcitonin gene-related peptide: a neurotrophic factor in the maintenance of acetylcholinesterase molecular forms in adult skeletal muscles. Brain Res 844(1–2):83–97. https://doi.org/10.1016/S0006-8993(99)01891-0
Fernandez HL, Hodges-Savola CA (1994) Axoplasmic transport of calcitonin gene-related peptide in rat peripheral nerve as function of age. Neurochem Res 19(11):1369–1377. https://doi.org/10.1007/bf00972465
Rossi SG, Dickerson IM, Rotundo RL (2003) Localization of the calcitonin gene-related peptide receptor complex at the vertebrate neuromuscular junction and its role in regulating acetylcholinesterase expression. J Biol Chem 278(27):24994–25000. https://doi.org/10.1074/jbc.M211379200
Chang C-P, Pearse RV II, O'Connell S, Rosenfeld MG (1993) Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 11(6):1187–1195. https://doi.org/10.1016/0896-6273(93)90230-O
McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N et al (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 393(6683):333–339. https://doi.org/10.1038/30666
Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM (2000) CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275(40):31438–31443. https://doi.org/10.1074/jbc.M005604200
Dickerson IM (2013) Role of CGRP-receptor component protein (RCP) in CLR/RAMP function. Curr Protein Pept Sci 14(5):407–415
Supowit SC, Katki KA, Hein TW, Gupta P, Kuo L, Dickerson IM, Dipette DJ (2011) Vascular reactivity to calcitonin gene-related peptide is enhanced in subtotal nephrectomy-salt induced hypertension. Am J Physiol Heart Circ Physiol 301:H683–H688
Prado MA, Evans-Bain B, Oliver KR, Dickerson IM (2001) The role of the CGRP-receptor component protein (RCP) in adrenomedullin receptor signal transduction. Peptides. 22(11):1773–1781 PubMed: 11754963
Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R (2003) Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 120(3):677–694 PubMed: 12895509
Caldero J, Casanovas A, Sorribas A, Esquerda JE (1992) Calcitonin gene-related peptide in rat spinal cord motoneurons: subcellular distribution and changes induced by axotomy. Neuroscience. 48(2):449–461. https://doi.org/10.1016/0306-4522(92)90504-u
Gharakhanlou R, Chadan S, Gardiner P (1999) Increased activity in the form of endurance training increases calcitonin gene-related peptide content in lumbar motoneuron cell bodies and in sciatic nerve in the rat. Neuroscience. 89(4):1229–1239. https://doi.org/10.1016/s0306-4522(98)00406-0
de Souza PA, Matheus SM, Castan EP, Campos DH, Cicogna AC, Carvalho RF et al (2011) Morphological aspects of neuromuscular junctions and gene expression of nicotinic acetylcholine receptors (nAChRs) in skeletal muscle of rats with heart failure. J Mol Histol 42(6):557–565. https://doi.org/10.1007/s10735-011-9354-2
Wang D, Wang X, Geng S, Bi Z (2015) Axonal regeneration in early stages of sciatic nerve crush injury is enhanced by α7nAChR in rats. Mol Biol Rep 42(3):603–609
Fagerlund MJ, Eriksson LI (2009) Current concepts in neuromuscular transmission. Br J Anaesth 103(1):108–114. https://doi.org/10.1093/bja/aep150
Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M et al (1994) GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 266(5187):1062–1064. https://doi.org/10.1126/science.7973664
Orenay-Boyacioglu S, Caliskan M, Boyacioglu O, Coskunoglu A, Bozkurt G, Cam FS (2019) Chronic tinnitus and BDNF/GDNF CpG promoter methylations: a case–control study. Mol Biol Rep 46(4):3929–3936. https://doi.org/10.1007/s11033-019-04837-0
Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD (2001) Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci 21(16):6136–6146
Zwick M, Teng L, Mu X, Springer JE, Davis BM (2001) Overexpression of GDNF induces and maintains hyperinnervation of muscle fibers and multiple end-plate formation. Exp Neurol 171(2):342–350. https://doi.org/10.1006/exnr.2001.7753
Gyorkos AM, McCullough MJ, Spitsbergen JM (2014) Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience 257:111–118. https://doi.org/10.1016/j.neuroscience.2013.10.068
Parnow A, Gharakhanlou R, Gorginkaraji Z, Rajabi S, Eslami R, Hedayati M et al (2012) Effects of endurance and resistance training on calcitonin gene-related peptide and acetylcholine receptor at slow and fast twitch skeletal muscles and sciatic nerve in male wistar rats. Int J Pept 2012. https://doi.org/10.1155/2012/962651
Deschenes MR, Sherman EG, Roby MA, Glass EK, Harris MB (2015) Effect of resistance training on neuromuscular junctions of young and aged muscles featuring different recruitment patterns. J Neurosci Res 93(3):504–513. https://doi.org/10.1002/jnr.23495
Deschenes MR, Kressin KA, Garratt RN, Leathrum CM, Shaffrey EC (2016) Effects of exercise training on neuromuscular junction morphology and pre- to post-synaptic coupling in young and aged rats. Neuroscience 316:167–177. https://doi.org/10.1016/j.neuroscience.2015.12.004
Deschenes MR, Li S, Adan MA, Oh JJ, Ramsey HC (2018) Muscle fibers and their synapses differentially adapt to aging and endurance training. Exp Gerontol 106:183–191. https://doi.org/10.1016/j.exger.2018.03.010
Polomoshnov D (2017) Acute HIT session induced changes and recovery in muscle activation level, voluntary force production and jump performance during 8 weeks of HIT training in recreationally endurance trained men. Master’s Thesis, University of Jyväskylä, pp 57–58
Milanović Z, Sporiš G, Weston M (2015) Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO 2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 45(10):1469–1481. https://doi.org/10.1007/s40279-015-0365-0
Cipryan L (2017) IL-6, antioxidant capacity and muscle damage markers following high-intensity interval training protocols. J Hum Kinet 56(1):139–148. https://doi.org/10.1515/hukin-2017-0031
Gorzi A, Rajabi H, Gharakhanlou R, Azad A (2013) Effects of endurance training on a12 acetyl cholinesterase activity in fast and slow-twitch skeletal muscles of male wistar rats. http://zjrms.ir/article-1-2371-en.html
Shepherd R, Gollnick P (1976) Oxygen uptake of rats at different work intensities. Pflugers Arch 362(3):219–222. https://doi.org/10.1007/bf00581173
Gorzi A, Taherkhani L, Rahmani A (2017) Effect of folate supplementation during 10 weeks of HIIT on serum levels of ghrelin and leptin in male Wistar rats. SJKU 22(5):13–21
Copp SW, Hirai DM, Musch TI, Poole DC (2010) Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol 588(Pt 24):5077–5087. https://doi.org/10.1113/jphysiol.2010.198382
Armstrong RB, Phelps RO (1984) Muscle fiber type composition of the rat hindlimb. Am J Anat 171(3):259–272. https://doi.org/10.1002/aja.1001710303
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29(9):e45-e. https://doi.org/10.1093/nar/29.9.e45
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):e36-e. https://doi.org/10.1093/nar/30.9.e36
Esfarjani F, Laursen PB (2007) Manipulating high-intensity interval training: effects on V˙ O2max, the lactate threshold and 3000 m running performance in moderately trained males. J Sci Med Sport 10(1):27–35. https://doi.org/10.1016/j.jsams.2006.05.014
Buchheit M, Laursen PB (2013) High-intensity interval training, solutions to the programming puzzle. Sports Med 43(10):927–954. https://doi.org/10.1007/s40279-013-0029-x
Obradović J, Vukadinović M, Pantović M, Baić M (2016) HIIT vs moderate intensity endurance training: impact on aerobic parameters in young adult men. Acta Kinesiologica 10(Suppl. 1):35–40
Ní Chéilleachair NJ, Harrison AJ, Warrington GD (2017) HIIT enhances endurance performance and aerobic characteristics more than high-volume training in trained rowers. J Sports Sci 35(11):1052–1058. https://doi.org/10.1080/02640414.2016.1209539
Blanco CE, Popper P, Micevych P (1997) α-CGRP mRNA levels in motoneurons innervating specific rat muscles. Mol Brain Res 44(2):253–261. https://doi.org/10.1016/s0169-328x(96)00227-6
McKenzie MJ, Goldfarb AH, Kump DS (2011) Gene response of the gastrocnemius and soleus muscles to an acute aerobic run in rats. J Sports Sci Med 10(2):385–392
Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA et al (1994) Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Phys 266(2 Pt 2):R375–R380. https://doi.org/10.1152/ajpregu.1994.266.2.R375
Röckl KS, Witczak CA, Goodyear LJ (2008) Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life 60(3):145–153. https://doi.org/10.1002/iub.21
McCullough MJ, Peplinski NG, Kinnell KR, Spitsbergen JM (2011) Glial cell line-derived neurotrophic factor protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience. 174:234–244. https://doi.org/10.1016/j.neuroscience.2010.11.016
Granata C, Oliveira RS, Little JP, Renner K, Bishop DJ (2017) Sprint-interval but not continuous exercise increases PGC-1α protein content and p53 phosphorylation in nuclear fractions of human skeletal muscle. Sci Rep 7:44227. https://doi.org/10.1038/srep44227
Gist NH, Fedewa MV, Dishman RK, Cureton KJ (2014) Sprint interval training effects on aerobic capacity: a systematic review and meta-analysis. Sports Med 44(2):269–279. https://doi.org/10.1007/s40279-013-0115-0.
Raleigh JP, Giles MD, Islam H, Nelms M, Bentley RF, Jones JH et al (2018) Contribution of central and peripheral adaptations to changes in maximal oxygen uptake following 4 weeks of sprint interval training. Appl Physiol Nutr Metab 43(10):1059–1068. https://doi.org/10.1139/apnm-2017-0864.
Gibala MJ, Bostad W, McCarthy DG (2019) Physiological adaptations to interval training to promote endurance. Curr Opin Physiol. https://doi.org/10.1016/j.cophys.2019.05.013
Acknowledgements
Part of this project (qPCR analysis) was performed at the Division of Clinical Physiology, Department of Laboratory Medicine, Karolinska Institutet (KI), SWEDEN. We thank Professor Brun Ulfhake, Dr. Mikael Altun, and Professor Thomas Gustafsson. We would also like to thank Professor Dr. Enrico Gori for his help in the final analysis of the text.
Funding
Firooz Jamshidi has received research grants from the University of Zanjan. Ali Gorzi’s works on KI were supported by funding from Karolinska Institute-Sweden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Institutional Animal Ethics Committee approved this work of the University of Zanjan, and all guidelines were followed (protocol code: ZNU.ECRA.2018–1). The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gorzi, A., Jamshidi, F., Rahmani, A. et al. Muscle gene expression of CGRP-α, CGRP receptor, nAchR-β, and GDNF in response to different endurance training protocols of Wistar rats. Mol Biol Rep 47, 5305–5314 (2020). https://doi.org/10.1007/s11033-020-05610-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05610-4