Abstract
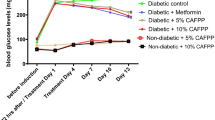
Type 2 diabetes mellitus (T2DM) is a metabolic disorder caused due to varied genetic and lifestyle factors. The search for a potential natural compound to enhance the treatment of diabetes is the need of the hour. Butein, a flavonoid, found sufficiently in Faba bean, is said to possess an anti-diabetic property. In-silico analysis, Butein is predicted as a potential anti-diabetic compound, due to its regulatory action on PPAR-Gamma. Based on this evidence, the Butein’s anti-diabetic action is studied in diabetic induced rat models. The drug property of Butein is studied through in-silico analysis to determine the metabolic properties. In animal models, the biochemical analysis, histopathological and gene expression against PPAR-Gamma were studied comparatively. Butein being a hydrophobic compound, the bioavailability is said to be minimum. Hence, Butein formulation was made using biopolymer Chitosan for the synergistic anti-diabetic action. The Butein Chitosan formulation was optimized and characterized using analytical techniques. Further, the anti-diabetic activity of Butein and Butein Chitosan formulation was studied in diabetic induced rats. The obtained in-silico analysis results showed that Butein is the most favorable drug. Apparently, in the rat model, Butein and Butein Chitosan formulation effectively controlled the blood glucose levels without any side effects. The histopathological observations of the tissue samples showed nontoxic activity. Additionally, the gene expression analysis predicted the possible mechanism of anti-diabetic action exhibited through the down regulation of PPAR-Gamma. Whereas, the Butein Chitosan formulation failed, to show synergetic anti-diabetic activity as expected. This study is vital in introducing Butein as a safe anti-diabetic compound, which can be used in the treatment of T2DM.





Similar content being viewed by others
References
Cho NH, Shaw JE, Karuranga S et al (2018) IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281. https://doi.org/10.1016/j.diabres.2018.02.023
Calderón-Salinas JV, Muñoz-Reyes EG, Guerrero-Romero JF et al (2011) Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol Cell Biochem 357:171–179. https://doi.org/10.1007/s11010-011-0887-1
Ahlqvist E, Van Zuydam NR, Groop LC, McCarthy MI (2015) The genetics of diabetic complications. Nat Rev Nephrol 11:277–287
Seko Y, Sumida Y, Tanaka S et al (2018) Insulin resistance increases the risk of incident type 2 diabetes mellitus in patients with non-alcoholic fatty liver disease. Hepatol Res 48:E42–E51. https://doi.org/10.1111/hepr.12925
Rani R, Dahiya S, Dhingra D et al (2017) European journal of pharmaceutical sciences evaluation of anti-diabetic activity of glycyrrhizin-loaded nanoparticles in nicotinamide-streptozotocin-induced diabetic rats. Eur J Pharm Sci 106:220–230. https://doi.org/10.1016/j.ejps.2017.05.068
Patel B, Oza B, Patel K et al (2013) Pattern of antidiabetic drugs use in type-2 diabetic patients in a medicine outpatient clinic of a tertiary care teaching hospital. Int J Basic Clin Pharmacol 2:485. https://doi.org/10.5455/2319-2003.ijbcp20130826
Azoulay L, Filion KB, Platt RW et al (2016) Incretin based drugs and the risk of pancreatic cancer: International multicentre cohort study. BMJ. https://doi.org/10.1136/bmj.i581
Häggström C, Van Hemelrijck M, Zethelius B et al (2017) Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. Int J Cancer 140:611–617. https://doi.org/10.1002/ijc.30480
Bahmani M, Zargaran A, Rafieian-Kopaei M, Saki K (2014) Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac J Trop Med 7:S348–S354. https://doi.org/10.1016/S1995-7645(14)60257-1
James P, Davis SP, Ravisankar V et al (2017) Novel antidiabetic molecules from the medicinal plants of Western Ghats of India, identified through wide-spectrum in silico analyses. J Herbs Spices Med Plants 23:249–262. https://doi.org/10.1080/10496475.2017.1315675
Xie JH, Jin ML, Morris GA et al (2016) Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr 56:S60–S84. https://doi.org/10.1080/10408398.2015.1069255
Kooti W, Farokhipour M, Asadzadeh Z et al (2016) The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Phys 8:1832–1842
Madar Z, Stark AH (2002) New legume sources as therapeutic agents. Br J Nutr 88:287–292. https://doi.org/10.1079/bjn2002719
Abd El-Maksoud H, Hussein MA, Kassem A (2013) Biochemical effect of vicine and divicine extracted from fava beans (vicia faba) in rats. Benha Vet Med J 24:98–110
Lin PY, Lai HM (2006) Bioactive compounds in legumes and their germinated products. J Agric Food Chem 54:3807–3814. https://doi.org/10.1021/jf060002o
Siddhuraju P, Manian S (2007) The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. Food Chem 105:950–958. https://doi.org/10.1016/j.foodchem.2007.04.040
Muzquiz M, Varela A, Burbano C et al (2012) Bioactive compounds in legumes: Pronutritive and antinutritive actions. implications for nutrition and health. Phytochem Rev 11:227–244. https://doi.org/10.1007/s11101-012-9233-9
Vessal M, Hemmati M, Vasei M (2003) Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C 135C:357–364. https://doi.org/10.1016/s1532-0456(03)00140-6
Baginsky C, Peña-Neiraálvaro CA et al (2013) Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile. J Food Compos Anal 31:1–6. https://doi.org/10.1016/j.jfca.2013.02.003
El-Mergawi R, Taie HAA (2014) Phenolic composition and antioxidant activity of raw seeds, green seeds and sprouts of ten faba bean (Vicia faba) cultivars consumed in Egypt. Int J Pharm Biol Sci
Kumar A, Nidhi PN, Sinha SK (2014) Nutritional and antinutritional attributes of faba bean (Vicia faba L.) germplasms growing in Bihar. India Physiol Mol Biol Plants 21:159–162. https://doi.org/10.1007/s12298-014-0270-2
Semwal RB, Semwal DK, Combrinck S, Viljoen A (2015) Butein: from ancient traditional remedy to modern nutraceutical. Phytochem Lett 11:188–201. https://doi.org/10.1016/j.phytol.2014.12.014
Prabhu DS, Rajeswari VD (2018) In vitro and in silico analyses of Vicia faba L. on peroxisome proliferator–activated receptor gamma. J Cell Biochem 119:7729–7737. https://doi.org/10.1002/jcb.27123
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. https://doi.org/10.1038/srep42717
Maunz A, Gütlein M, Rautenberg M et al (2013) Lazar: a modular predictive toxicology framework. Front Pharmacol 4:38. https://doi.org/10.3389/fphar.2013.00038
Masalova O, Kulikouskaya V, Shutava T, Agabekov V (2013) Alginate and chitosan gel nanoparticles for efficient protein entrapment. In: Physics procedia, pp 69–75
Chandy T, Mooradian DL, Rao GHR (1998) Chitosan/polyethylene glycol-alginate microcapsules for oral delivery of hirudin. J Appl Polym Sci 70:2143–2153
Radhakrishnan A, Jose GM, Kurup M (2015) PEG-penetrated chitosan–alginate co-polysaccharide-based partially and fully cross-linked hydrogels as ECM mimic for tissue engineering applications. Prog Biomater 4:101–112. https://doi.org/10.1007/s40204-015-0041-3
Kamboj S, Saini V, Bala S (2014) Formulation and characterization of drug loaded nonionic surfactant vesicles (Niosomes) for oral bioavailability enhancement. Sci World J. https://doi.org/10.1155/2014/959741
Masiello P, Broca C, Gross R et al (1998) Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 47:224–229. https://doi.org/10.2337/diab.47.2.224
Szkudelski T (2012) Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med 237:481–490
Prabhu S, Vinodhini S, Elanchezhiyan C, Rajeswari D (2018) Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J Diabetes 10:28–42. https://doi.org/10.1111/1753-0407.12554
Prabhu DS, Rajeswari VD (2018) In vitro and in silico analyses of Vicia faba L. on Peroxisome proliferator-activated receptor gamma. J Cell Biochem 119:7729–7737. https://doi.org/10.1002/jcb.27123
International Diabetes Federation - Home. https://idf.org/. Accessed 20 Jan 2020
Paul E, Cherniack EP (2010) The potential influence of plant polyphenols on the aging process. Forsch Komplementmed 17:181–187. https://doi.org/10.1159/000319143
Sharma M, Sharma R, Jain DK (2016) Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica (Cairo)
Uppal S, Italiya KS, Chitkara D, Mittal A (2018) Nanoparticulate-based drug delivery systems for small molecule anti-diabetic drugs: an emerging paradigm for effective therapy. Acta Mater Inc
Campos EJ, Campos A, Martins J, Ambrósio AF (2017) Opening eyes to nanomedicine: where we are, challenges and expectations on nanotherapy for diabetic retinopathy. Nanomed Nanotechnol Biol Med 13:2101–2113. https://doi.org/10.1016/j.nano.2017.04.008
Biradar S, Ravichandran P, Gopikrishnan R, et al (2011) Calcium carbonate nanoparticles: synthesis, characterization and biocompatibility. J Nanosci Nanotechnol. pp 6868–6874
Woitiski CB, Neufeld RJ, Veiga F et al (2010) Pharmacological effect of orally delivered insulin facilitated by multilayered stable nanoparticles. Eur J Pharm Sci 41:556–563. https://doi.org/10.1016/j.ejps.2010.08.009
Arunagiri A, Safdar R, Thanabalan M et al (2018) Potential of Chitosan and its derivatives for controlled drug release applications—a review. J Drug Deliv Sci Technol 49:642–659. https://doi.org/10.1016/j.jddst.2018.10.020
Wong CY, Al-Salami H, Dass CR (2018) Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. Int J Pharm 537:223–244. https://doi.org/10.1016/j.ijpharm.2017.12.036
Patel DK, Kumar R, Laloo D, Hemalatha S (2012) Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac J Trop Biomed 2:411–420
Mahapatra DK, Asati V, Bharti SK (2015) Chalcones and their therapeutic targets for the management of diabetes: structural and pharmacological perspectives. Eur J Med Chem 92:839–865
Hasani-Ranjbar S, Jouyandeh Z, Abdollahi M (2013) A systematic review of anti-obesity medicinal plants—an update. J Diabetes Metab Disord 12:1. https://doi.org/10.1186/2251-6581-12-28
Song NJ, Yoon HJ, Kim KH et al (2013) Butein is a novel anti-adipogenic compound. J Lipid Res 54:1385–1396. https://doi.org/10.1194/jlr.M035576
Larsen TM, Toubro S, Astrup A (2003) PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes 27:147–161
Barroso I, Gurnell M, Crowley VEF et al (1999) Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402:880–883. https://doi.org/10.1038/47254
Padmavathi G, Roy NK, Bordoloi D et al (2017) Butein in health and disease: a comprehensive review. Phytomedicine 25:118–127
Ahad A, Mujeeb M, Ahsan H, Siddiqui WA (2014) Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie 106:101–110. https://doi.org/10.1016/j.biochi.2014.08.006
Gong Z, Huang C, Sheng X et al (2009) The role of tanshinone IIA in the treatment of obesity through peroxisome proliferator-activated receptor γ antagonism. Endocrinology 150:104–113. https://doi.org/10.1210/en.2008-0322
Frkic RL, Marshall AC, Blayo AL et al (2018) PPARγ in complex with an antagonist and inverse agonist: a tumble and trap mechanism of the activation helix. IScience 5:69–79. https://doi.org/10.1016/j.isci.2018.06.012
Acknowledgements
The author thanks VIT for providing all the research facilities and support to carry out the research process.
Funding
No funding source is available.
Author information
Authors and Affiliations
Contributions
All authors equally contributed and gave their final approval to the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
The study is approved by the Institutional Animal Ethics Committee (IAEC) at VIT (VIT/IAEC/14th/Nov4/15). The study is carried out according to the guidelines advocated by the Committee for the Purpose of Supervision and Control of Experiments on Animals (CPSCEA), Government of India.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prabhu, D.S., Rajeswari, V.D. PPAR-Gamma as putative gene target involved in Butein mediated anti-diabetic effect. Mol Biol Rep 47, 5273–5283 (2020). https://doi.org/10.1007/s11033-020-05605-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05605-1