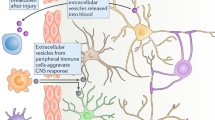
Abstract
Stroke is induced by a partial disruption of cerebral blood flow to the brain and is related to high morbidity and mortality. In the central nervous system, exosomes have been proven to exert neuroprotective effects, reducing brain damage following a stroke. This review was performed by searching the relevant articles in the SCIENCEDIRECT, PUBMED, and Web of Science databases from respective inception to November 2018. We review the relationship between exosomes and angiogenesis, neurogenesis, antiapoptosis, autophagy, and the blood–brain barrier in stroke. Moreover, exosomes are found to be a promising tool for the diagnosis and treatment of stroke. In summary, exosomes provide a novel way to alleviate brain damage following a stroke.


Similar content being viewed by others
References
Yan T, Chopp M, Chen J (2015) Experimental animal models and inflammatory cellular changes in cerebral ischemic and hemorrhagic stroke. Neurosci Bull 31(6):717–734. https://doi.org/10.1007/s12264-015-1567-z
Venkat P, Chen J, Chopp M (2018) Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab 38(12):2165–2178. https://doi.org/10.1177/0271678X18782789
Xiao Y, Geng F, Wang G, Li X, Zhu J, Zhu W (2018) Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J Cell Biochem. https://doi.org/10.1002/jcb.27519
Osier N, Motamedi V, Edwards K, Puccio A, Diaz-Arrastia R, Kenney K, Gill J (2018) Exosomes in acquired neurological disorders: new insights into pathophysiology and treatment. Mol Neurobiol 55(12):9280–9293. https://doi.org/10.1007/s12035-018-1054-4
Zhang ZG, Chopp M (2015) Promoting brain remodeling to aid in stroke recovery. Trends Mol Med 21(9):543–548. https://doi.org/10.1016/j.molmed.2015.07.005
Properzi F, Ferroni E, Poleggi A, Vinci R (2015) The regulation of exosome function in the CNS: implications for neurodegeneration. Swiss Med Wkly 145:w14204. https://doi.org/10.4414/smw.2015.14204
Zhang ZG, Chopp M (2016) Exosomes in stroke pathogenesis and therapy. J Clin Invest 126(4):1190–1197. https://doi.org/10.1172/JCI81133
Sun B, Peng J, Wang S, Liu X, Zhang K, Zhang Z, Wang C, Jing X, Zhou C, Wang Y (2018) Applications of stem cell-derived exosomes in tissue engineering and neurological diseases. Rev Neurosci 29(5):531–546. https://doi.org/10.1515/revneuro-2017-0059
Kawikova I, Askenase PW (2015) Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res 1617:63–71. https://doi.org/10.1016/j.brainres.2014.09.070
Wang W, Li Z, Feng J (2018) The potential role of exosomes in the diagnosis and therapy of ischemic diseases. Cytotherapy 20(10):1204–1219. https://doi.org/10.1016/j.jcyt.2018.06.012
Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31(4):642–648. https://doi.org/10.1016/j.mcn.2005.12.003
An K, Klyubin I, Kim Y, Jung JH, Mably AJ, O'Dowd ST, Lynch T, Kanmert D, Lemere CA, Finan GM, Park JW, Kim TW, Walsh DM, Rowan MJ, Kim JH (2013) Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol Brain 6:47. https://doi.org/10.1186/1756-6606-6-47
Tang BL (2018) Promoting axonal regeneration through exosomes: An update of recent findings on exosomal PTEN and mTOR modifiers. Brain Res Bull 143:123–131. https://doi.org/10.1016/j.brainresbull.2018.10.008
Yuyama K, Igarashi Y (2016) Physiological and pathological roles of exosomes in the nervous system. Biomol Concepts 7(1):53–68. https://doi.org/10.1515/bmc-2015-0033
Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R (2014) Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 3:24722. https://doi.org/10.3402/jev.v3.24722
Frohlich D, Kuo WP, Fruhbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J, Kramer-Albers EM (2014) Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2013.0510
Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R (2012) Emerging role of neuronal exosomes in the central nervous system. Front Physiol 3:145. https://doi.org/10.3389/fphys.2012.00145
Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM (2013) Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11(7):e1001604. https://doi.org/10.1371/journal.pbio.1001604
Braccioli L, van Velthoven C, Heijnen CJ (2014) Exosomes: a new weapon to treat the central nervous system. Mol Neurobiol 49(1):113–119. https://doi.org/10.1007/s12035-013-8504-9
Huang J, Kang B, Qu Y, Mu D (2017) Protective effect of exosome on organs after ischemia-reperfusion injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 31(6):751–754. https://doi.org/10.7507/1002-1892.201701104
Malm T, Loppi S, Kanninen KM (2016) Exosomes in Alzheimer's disease. Neurochem Int 97:193–199. https://doi.org/10.1016/j.neuint.2016.04.011
Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J (2017) The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. https://doi.org/10.3390/ijms18061153
Russell AE, Jun S, Sarkar S, Geldenhuys WJ, Lewis SE, Rellick SL, Simpkins JW (2019) Extracellular vesicles secreted in response to cytokine exposure increase mitochondrial oxygen consumption in recipient cells. Front Cell Neurosci 13:51. https://doi.org/10.3389/fncel.2019.00051
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H (2018) New technologies for analysis of extracellular vesicles. Chem Rev 118(4):1917–1950. https://doi.org/10.1021/acs.chemrev.7b00534
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383. https://doi.org/10.1083/jcb.201211138
Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Morhayim J, Rudjito R, van Leeuwen JP, van Driel M (2016) Paracrine signaling by extracellular vesicles via osteoblasts. Curr Mol Biol Rep 2:48–55. https://doi.org/10.1007/s40610-016-0034-6
Russell AE, Sneider A, Witwer KW, Bergese P, Bhattacharyya SN, Cocks A, Cocucci E, Erdbrugger U, Falcon-Perez JM, Freeman DW, Gallagher TM, Hu S, Huang Y, Jay SM, Kano SI, Lavieu G, Leszczynska A, Llorente AM, Lu Q, Mahairaki V, Muth DC, Noren Hooten N, Ostrowski M, Prada I, Sahoo S, Schoyen TH, Sheng L, Tesch D, Van Niel G, Vandenbroucke RE, Verweij FJ, Villar AV, Wauben M, Wehman AM, Yin H, Carter DRF, Vader P (2019) Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J Extracell Vesicles 8(1):1684862. https://doi.org/10.1080/20013078.2019.1684862
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ (2006) Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20(5):847–856. https://doi.org/10.1038/sj.leu.2404132
Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS Jr, Olson SD (2018) Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells 36(1):79–90. https://doi.org/10.1002/stem.2730
Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC (2018) Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transplant 27(3):349–363. https://doi.org/10.1177/0963689717723636
Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J (2018) Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther 9(1):320. https://doi.org/10.1186/s13287-018-1069-9
Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G (2007) Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110(7):2440–2448. https://doi.org/10.1182/blood-2007-03-078709
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659. https://doi.org/10.1038/ncb1596
Ratajczak MZ, Ratajczak J (2016) Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin Transl Med 5(1):7. https://doi.org/10.1186/s40169-016-0087-4
Yates AG, Anthony DC, Ruitenberg MJ, Couch Y (2019) Systemic immune response to traumatic CNS injuries-are extracellular vesicles the missing link? Front Immunol 10:2723. https://doi.org/10.3389/fimmu.2019.02723
Mahdavipour M, Hassanzadeh G, Seifali E, Mortezaee K, Aligholi H, Shekari F, Sarkoohi P, Zeraatpisheh Z, Nazari A, Movassaghi S, Akbari M (2019) Effects of neural stem cell-derived extracellular vesicles on neuronal protection and functional recovery in the rat model of middle cerebral artery occlusion. Cell Biochem Funct. https://doi.org/10.1002/cbf.3484
Yang Y, Boza-Serrano A, Dunning CJR, Clausen BH, Lambertsen KL, Deierborg T (2018) Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflammation 15(1):168. https://doi.org/10.1186/s12974-018-1204-7
Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL (2018) Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke 49(5):1248–1256. https://doi.org/10.1161/STROKEAHA.117.020353
Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL (2020) Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience 42(1):1–17. https://doi.org/10.1007/s11357-019-00115-w
Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B (2019) Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation 16(1):216. https://doi.org/10.1186/s12974-019-1602-5
Bang OY, Kim EH (2019) Mesenchymal stem cell-derived extracellular vesicle therapy for stroke: challenges and progress. Front Neurol 10:211. https://doi.org/10.3389/fneur.2019.00211
Kodali M, Castro OW, Kim DK, Thomas A, Shuai B, Attaluri S, Upadhya R, Gitai D, Madhu LN, Prockop DJ, Shetty AK (2019) Intranasally administered human MSC-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int J Mol Sci. https://doi.org/10.3390/ijms21010181
van Niel G, D'Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19(4):213–228. https://doi.org/10.1038/nrm.2017.125
Piper RC, Katzmann DJ (2007) Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23:519–547. https://doi.org/10.1146/annurev.cellbio.23.090506.123319
Luzio JP, Gray SR, Bright NA (2010) Endosome-lysosome fusion. Biochem Soc Trans 38(6):1413–1416. https://doi.org/10.1042/BST0381413
Thery C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3:15. https://doi.org/10.3410/B3-15
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH (2019) Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. https://doi.org/10.3390/cells8040307
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345. https://doi.org/10.1038/nbt.1807
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35(7):2383–2390. https://doi.org/10.1016/j.biomaterials.2013.11.083
Simpson RJ, Jensen SS, Lim JW (2008) Proteomic profiling of exosomes: current perspectives. Proteomics 8(19):4083–4099. https://doi.org/10.1002/pmic.200800109
Properzi F, Logozzi M, Fais S (2013) Exosomes: the future of biomarkers in medicine. Biomark Med 7(5):769–778. https://doi.org/10.2217/bmm.13.63
Peterson MF, Otoc N, Sethi JK, Gupta A, Antes TJ (2015) Integrated systems for exosome investigation. Methods 87:31–45. https://doi.org/10.1016/j.ymeth.2015.04.015
Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, Harrison P, Sargent IL (2011) Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7(6):780–788. https://doi.org/10.1016/j.nano.2011.04.003
Manuel GE, Johnson T, Liu D (2017) Therapeutic angiogenesis of exosomes for ischemic stroke. Int J Physiol Pathophysiol Pharmacol 9(6):188–191
Shen H, Yao X, Li H, Li X, Zhang T, Sun Q, Ji C, Chen G (2018) Role of exosomes derived from miR-133b modified MSCs in an experimental rat model of intracerebral hemorrhage. J Mol Neurosci 64(3):421–430. https://doi.org/10.1007/s12031-018-1041-2
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150:137–149. https://doi.org/10.1016/j.biomaterials.2017.10.012
Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M (2017) Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8(28):45200–45212. https://doi.org/10.18632/oncotarget.16778
Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, Du JL (2017) Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res 27(7):882–897. https://doi.org/10.1038/cr.2017.62
Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y (2015) Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122(4):856–867. https://doi.org/10.3171/2014.11.JNS14770
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7):1556–1564. https://doi.org/10.1002/stem.1129
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M (2013) MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31(12):2737–2746. https://doi.org/10.1002/stem.1409
Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD (2018) Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J 32(2):654–668. https://doi.org/10.1096/fj.201700600R
Venkat P, Chopp M, Chen J (2018) Cell-based and exosome therapy in diabetic stroke. Stem Cells Transl Med 7(6):451–455. https://doi.org/10.1002/sctm.18-0014
Qing L, Chen H, Tang J, Jia X (2018) Exosomes and their microRNA cargo: new players in peripheral nerve regeneration. Neurorehabil Neural Repair 32(9):765–776. https://doi.org/10.1177/1545968318798955
Yang Y, Ye Y, Su X, He J, Bai W, He X (2017) MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci 11:55. https://doi.org/10.3389/fncel.2017.00055
Deng M, Xiao H, Peng H, Yuan H, Xu Y, Zhang G, Tang J, Hu Z (2018) Preservation of neuronal functions by exosomes derived from different human neural cell types under ischemic conditions. Eur J Neurosci 47(2):150–157. https://doi.org/10.1111/ejn.13784
de Rivero Vaccari JP, Brand F 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, Bullock MR, Dietrich WD, Keane RW (2016) Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem 136(Suppl 1):39–48. https://doi.org/10.1111/jnc.13036
Ojha CR, Lapierre J, Rodriguez M, Dever SM, Zadeh MA, DeMarino C, Pleet ML, Kashanchi F, El-Hage N (2017) Interplay between autophagy, exosomes and HIV-1 associated neurological disorders: new insights for diagnosis and therapeutic applications. Viruses. https://doi.org/10.3390/v9070176
Huang JL, Qu Y, Tang J, Zou R, Li SP, Li YF, Zhang L, Xia B, Mu DZ (2018) Protective effect of astrocyte exosomes on hypoxic-ischemic neurons. Zhongguo Dang Dai Er Ke Za Zhi 20(5):397–402
Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, Zhang ZG, Chopp M (2017) Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant 26(2):243–257. https://doi.org/10.3727/096368916X693031
Lafourcade C, Ramirez JP, Luarte A, Fernandez A, Wyneken U (2016) MiRNAs in astrocyte-derived exosomes as possible mediators of neuronal plasticity. J Exp Neurosci 10(Suppl 1):1–9. https://doi.org/10.4137/JEN.S39916
Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R (2016) Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 64(6):896–910. https://doi.org/10.1002/glia.22963
Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, Schachner M, Kleene R (2011) Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci 31(20):7275–7290. https://doi.org/10.1523/JNEUROSCI.6476-10.2011
Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, Wu D, Zhang ZG (2017) Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol Neurobiol 54(4):2659–2673. https://doi.org/10.1007/s12035-016-9851-0
Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M (2017) MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48(3):747–753. https://doi.org/10.1161/STROKEAHA.116.015204
Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM, Guan J, Bao XJ, Wu H, Han Q, Wang RZ, Zhao CH (2016) Protection of nerve injury with exosome extracted from mesenchymal stem cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38(1):33–36. https://doi.org/10.3881/j.issn.1000-503X.2016.01.006
Hira K, Ueno Y, Tanaka R, Miyamoto N, Yamashiro K, Inaba T, Urabe T, Okano H, Hattori N (2018) Astrocyte-derived exosomes treated with a semaphorin 3A inhibitor enhance stroke recovery via prostaglandin D2 synthase. Stroke 49(10):2483–2494. https://doi.org/10.1161/STROKEAHA.118.021272
Bahrini I, Song JH, Diez D, Hanayama R (2015) Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep 5:7989. https://doi.org/10.1038/srep07989
Yue Y, Qu Y, Mu DZ (2017) Research advances in mesenchymal stem cell-derived exosomes in treatment of brain injury. Zhongguo Dang Dai Er Ke Za Zhi 19(12):1285–1290
Huang X, Ding J, Li Y, Liu W, Ji J, Wang H, Wang X (2018) Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res 371(1):269–277. https://doi.org/10.1016/j.yexcr.2018.08.021
Xiao B, Chai Y, Lv S, Ye M, Wu M, Xie L, Fan Y, Zhu X, Gao Z (2017) Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med 40(4):1201–1209. https://doi.org/10.3892/ijmm.2017.3106
Tassew NG, Charish J, Shabanzadeh AP, Luga V, Harada H, Farhani N, D'Onofrio P, Choi B, Ellabban A, Nickerson PEB, Wallace VA, Koeberle PD, Wrana JL, Monnier PP (2017) Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep 20(1):99–111. https://doi.org/10.1016/j.celrep.2017.06.009
Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX (2017) Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem 43(1):52–68. https://doi.org/10.1159/000480317
Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L (2018) Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem 47(2):864–878. https://doi.org/10.1159/000490078
Otero-Ortega L, Laso-Garcia F, Gomez-de Frutos M, Fuentes B, Diekhorst L, Diez-Tejedor E, Gutierrez-Fernandez M (2019) Role of exosomes as a treatment and potential biomarker for stroke. Transl Stroke Res 10(3):241–249. https://doi.org/10.1007/s12975-018-0654-7
Tsilioni I, Panagiotidou S, Theoharides TC (2014) Exosomes in neurologic and psychiatric disorders. Clin Ther 36(6):882–888. https://doi.org/10.1016/j.clinthera.2014.05.005
Gao W, Li F, Liu L, Xu X, Zhang B, Wu Y, Yin D, Zhou S, Sun D, Huang Y, Zhang J (2018) Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp Neurol 307:99–108. https://doi.org/10.1016/j.expneurol.2018.06.001
Luarte A, Batiz LF, Wyneken U, Lafourcade C (2016) Potential therapies by stem cell-derived exosomes in CNS diseases: focusing on the neurogenic niche. Stem Cells Int 2016:5736059. https://doi.org/10.1155/2016/5736059
Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao H, Xu T, Chen L, Xu Y (2016) Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS ONE 11(9):e0163645. https://doi.org/10.1371/journal.pone.0163645
Li DB, Liu JL, Wang W, Li RY, Yu DJ, Lan XY, Li JP (2017) Plasma exosomal miR-422a and miR-125b-2-3p serve as biomarkers for ischemic stroke. Curr Neurovasc Res 14(4):330–337. https://doi.org/10.2174/1567202614666171005153434
Tang H, Wu H, Yang Y, Zhao J, Chen J (2015) Progress in study on the role of exosome-derived microRNA in diagnosis and treatment of diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban 40(11):1270–1275. https://doi.org/10.11817/j.issn.1672-7347.2015.11.018
Goetzl L, Merabova N, Darbinian N, Martirosyan D, Poletto E, Fugarolas K, Menkiti O (2018) Diagnostic potential of neural exosome cargo as biomarkers for acute brain injury. Ann Clin Transl Neurol 5(1):4–10. https://doi.org/10.1002/acn3.499
Chopp M, Zhang ZG (2015) Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opin Emerg Drugs 20(4):523–526. https://doi.org/10.1517/14728214.2015.1061993
Xiong Y, Mahmood A, Chopp M (2017) Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res 12(1):19–22. https://doi.org/10.4103/1673-5374.198966
Chen F, Du Y, Esposito E, Liu Y, Guo S, Wang X, Lo EH, Xing C, Ji X (2015) Effects of focal cerebral ischemia on exosomal versus serum miR126. Transl Stroke Res 6(6):478–484. https://doi.org/10.1007/s12975-015-0429-3
Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, Vazquez J, Diez-Tejedor E, Gutierrez-Fernandez M (2018) Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab 38(5):767–779. https://doi.org/10.1177/0271678X17708917
Ching RC, Wiberg M, Kingham PJ (2018) Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther 9(1):266. https://doi.org/10.1186/s13287-018-1017-8
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33(11):1711–1715. https://doi.org/10.1038/jcbfm.2013.152
Su SA, Xie Y, Fu Z, Wang Y, Wang JA, Xiang M (2017) Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget 8(15):25700–25712. https://doi.org/10.18632/oncotarget.14878
Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, Shao PL, Chen YL, Chai HT, Lin KC, Liu CF, Chang HW, Lee MS, Yip HK (2016) Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7(46):74537–74556. https://doi.org/10.18632/oncotarget.12902
Yang Y, Cai Y, Zhang Y, Liu J, Xu Z (2018) Exosomes secreted by adipose-derived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen-glucose deprivation in vitro through microRNA-181b/TRPM7 axis. J Mol Neurosci 65(1):74–83. https://doi.org/10.1007/s12031-018-1071-9
Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N (2016) Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol 79:360–369. https://doi.org/10.1016/j.biocel.2016.09.002
Yang J, Zhang X, Chen X, Wang L, Yang G (2017) Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 7:278–287. https://doi.org/10.1016/j.omtn.2017.04.010
Lapchak PA, Boitano PD, de Couto G, Marban E (2018) Intravenous xenogeneic human cardiosphere-derived cell extracellular vesicles (exosomes) improves behavioral function in small-clot embolized rabbits. Exp Neurol 307:109–117. https://doi.org/10.1016/j.expneurol.2018.06.007
Lopez-Leal R, Court FA (2016) Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol 36(3):429–436. https://doi.org/10.1007/s10571-015-0314-3
Kawahara H, Hanayama R (2018) The role of exosomes/extracellular vesicles in neural signal transduction. Biol Pharm Bull 41(8):1119–1125. https://doi.org/10.1248/bpb.b18-00167
Levy E (2017) Exosomes in the diseased brain: first insights from in vivo studies. Front Neurosci 11:142. https://doi.org/10.3389/fnins.2017.00142
Reza-Zaldivar EE, Hernandez-Sapiens MA, Minjarez B, Gutierrez-Mercado YK, Marquez-Aguirre AL, Canales-Aguirre AA (2018) Potential effects of MSC-derived exosomes in neuroplasticity in Alzheimer's disease. Front Cell Neurosci 12:317. https://doi.org/10.3389/fncel.2018.00317
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release 207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033
Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z (2015) Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37(6):2415–2424. https://doi.org/10.1159/000438594
Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H (2016) Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J Stem Cells 8(9):297–305. https://doi.org/10.4252/wjsc.v8.i9.297
Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM (2015) Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med 4(10):1131–1143. https://doi.org/10.5966/sctm.2015-0078
Otero-Ortega L, Laso-Garcia F, Gomez-de Frutos MD, Rodriguez-Frutos B, Pascual-Guerra J, Fuentes B, Diez-Tejedor E, Gutierrez-Fernandez M (2017) White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep 7:44433. https://doi.org/10.1038/srep44433
Dabrowska S, Andrzejewska A, Lukomska B, Janowski M (2019) Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation 16(1):178. https://doi.org/10.1186/s12974-019-1571-8
Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP, Cha JM, Bang OY (2019) Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and microRNA study. Transl Stroke Res 10(5):509–521. https://doi.org/10.1007/s12975-018-0668-1
Hong SB, Yang H, Manaenko A, Lu J, Mei Q, Hu Q (2019) Potential of exosomes for the treatment of stroke. Cell Transplant 28(6):662–670. https://doi.org/10.1177/0963689718816990
Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B (2017) Exosomes, an unmasked culprit in neurodegenerative diseases. Front Neurosci 11:26. https://doi.org/10.3389/fnins.2017.00026
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, MM., Feng, YS., Tan, ZX. et al. The role of exosomes in stroke. Mol Biol Rep 47, 6217–6228 (2020). https://doi.org/10.1007/s11033-020-05569-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05569-2