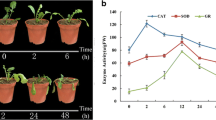
Abstract
Heat stress has a severe impact on potato growth and tuberization process, always resulting in the decrease of tuber yield and quality. Therefore, it is of great significance for potato breeding to illuminate the mechanism of heat stress on potato and explore heat resistant genes. In this study, two cDNA libraries from normal potato leaves (20 °C day/18 °C night) and potato leaves with 3 days of heat treatment (35 °C day/28 °C night) were constructed respectively. Totally, 1420 differentially expressed genes (DEGs) were identified. The expression patterns of 12 randomly selected genes detected using droplet digital PCR agreed with the sequencing data. Gene ontology analysis showed that these DEGs were clustered into 49 different GO types, reflecting the functional diversity of the heat stress response genes. The results of KEGG pathway enrichment showed the potential biological pathways in which the DEGs were involved, indicating that these pathways may be involved in heat tolerance regulation. Most potato heat transcription factors (StHsfs) and heat shock proteins (StHsps) were not expressed efficiently based on expression profile of these DEGs. StHsp26-CP and StHsp70 were markedly increased after 3 days of heat treatment. These data will be useful for further understanding the molecular mechanisms of potato plant tolerance to heat stress and provide a basis for breeding heat-tolerance varieties.






Similar content being viewed by others
References
Millam S (2006) Potato (Solanum tuberosum L.). In: Wang et al. (eds) Agrobacterium protocols Volume 2. Methods Mol Biol 344:25–35. https://doi.org/10.1007/978-1-4939-1658-0_8
Tang RM, Zhu WJ, Song XY, Lin XZ, Cai JH, Wang M, Yang Q (2016) Genome-wide identification and function analyses of heat shock transcription factors in potato. Front Plant Sci 7:490. https://doi.org/10.3389/fpls.2016.00490
Herman DJ, Knowles LO, Knowles NR (2017) Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta 245(3):563–582. https://doi.org/10.1007/s00425-016-2626-z
Rykaczewska K (2013) The impact of high temperature during growing season on potato cultivars with different response to environmental stresses. Am J Plant Sci 4:2386–2393. https://doi.org/10.4236/ajps.2013.412295
Lafta AM, Lorenzen JH (1995) Effect of high temperature on plant growth and carbohydrate metabolism in potato. Plant Physiol 109(2):637–643. https://doi.org/10.2307/4276846
Yan J, Yu L, Xuan J, Lu Y, Lu S, Zhu W (2016) De novo transcriptome sequencing and gene expression profiling of spinach (Spinacia oleracea L.) leaves under heat stress. Sci Rep 6:19473. https://doi.org/10.1038/srep19473
Tang RM, Niu SY, Zhang GD, Chen GS, Haroon M, Yang Q, Rajora OP, Li XQ (2018) Physiological and growth responses of potato cultivars to heat stress. Botany 96:897–912. https://doi.org/10.1139/cjb-2018-0125
Auffray C, Hood L (2012) Editorial: systems biology and personalized medicine—the future is now. Biotechnol J 7(8):938–939. https://doi.org/10.1002/biot.201200242
Mardis ER (2008) Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet 9:387–402. https://doi.org/10.1146/annurev.genom.9.081307.164359
Qi XH, Xu XW, Lin XJ, Zhang WJ, Chen XH (2012) Identification of differentially expressed genes in cucumber (Cucumis sativus L.) root under waterlogging stress by digital gene expression profile. Genomics 99(3):160–168. https://doi.org/10.1016/j.ygeno.2011.12.008
Zhou Y, Gao F, Liu R, Feng J, Li H (2012) De novo sequencing and analysis of root transcriptome using 454 pyrosequencing to discover putative genes associated with drought tolerance in Ammopiptanthus mongolicus. BMC Genomics 13:266. https://doi.org/10.1186/1471-2164-13-266
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134(4):1683–1696. https://doi.org/10.1104/pp.103.033431
Li YF, Wang YX, Tang YH, Kakani VG, Mahalingam R (2013) Transcriptome analysis of heat stress response in switchgrass (Panicum virgatum L.). BMC Plant Biol 13:153–164. https://doi.org/10.1186/1471-2229-13-153
Zhao P, Wang D, Wang R, Kong N, Zhang C, Yang C, Wu W, Ma H, Chen Q (2018) Genome-wide analysis of the potato Hsp20 gene family: identification, genomic organization and expression profiles in response to heat stress. BMC Genomics 19:61. https://doi.org/10.1186/s12864-018-4443-1
Mittal D, Chakrabarti S, Sarkar A, Singh A, Grover A (2009) Heat shock factor gene family in rice: genomic organization and transcript expression profiling in response to high temperature, low temperature and oxidative stresses. Plant Physiol Biochem 47(9):785–795. https://doi.org/10.1016/j.plaphy.2009.05.003
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21. https://doi.org/10.1093/bioinformatics/bts635
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106–R117. https://doi.org/10.1186/gb-2010-11-10-r106
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. https://doi.org/10.1093/bioinformatics/btp616
Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676. https://doi.org/10.1093/bioinformatics/bti610
Alexa A, Rahnenfuhrer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22:1600–1607. https://doi.org/10.1093/bioinformatics/btl140
Hald A (1960) The compound hypergeometric distribution and a system of single sampling inspection plans based on prior distributions and costs. Technometrics 2:275–340. https://doi.org/10.2307/1266247
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684. https://doi.org/10.3390/ijms14059643
Bethke PC, Nassar AMK, Kubow S, Leclerc YN, Li X, Haroon M, Molen T, Bamberg J, Martin M, Donnelly DJ (2014) History and origin of Russet Burbank (Netted Gem) a sport of Burbank. Am J Potato Res 91:594–609. https://doi.org/10.1007/s12230-014-9397-5
Ahuja I, Vos RCHD, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15(12):664–674. https://doi.org/10.1016/j.tplants.2010.08.002
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37(3):118–125. https://doi.org/10.1016/j.tibs.2011.11.007
Zhong L, Zhou W, Wang H, Ding S, Lu Q, Wen X, Peng L, Zhang L, Lu C (2013) Chloroplast small heat shock protein HSP21 interacts with plastid nucleoid protein pTAC5 and is essential for chloroplast development in Arabidopsis under heat stress. Plant Cell 25(8):2925–2943. https://doi.org/10.1105/tpc.113.111229
Ahmad P, Prasad MNV (2012) Chapter 1. In: Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York. https://doi.org/10.1007/978-1-4614-0815-4
Huang YC, Niu CY, Yang CR, Jinn TL (2016) The heat-stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol 172(2):1182–1199. https://doi.org/10.1104/pp.16.00860
Bale J, Masters G, Hodkinson I, Awmack C, Bezemer T, Brown V, Butterfield J, Buse A, Coulson J, Farrar J, Good J, Harrington R, Hartley S, Jones T, Lindroth R, Press M, Symrnioudis I, Watt A, Whittaker J (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8:1–16. https://doi.org/10.1046/j.1365-2486.2002.00451.x
Pandey P, Ramegowda V, Senthil-Kumar M (2015) Shared and unique responses of plants to multiple individual stresses and stress combinations: physiological and molecular mechanisms. Front Plant Sci 6:723. https://doi.org/10.3389/fpls.2015.00723
Bennett R, Wallsgrove R (1994) Secondary metabolites in plant defence mechanisms. N Physiol 127:617–633. https://doi.org/10.1111/j.1469-8137.1994.tb02968.x
Du Fall LA, Solomon PS (2011) Role of cereal secondary metabolites involved in mediating the outcome of plant–pathogen interactions. Metabolites 1:64–78. https://doi.org/10.3390/metabo1010064
Guo M, Lu JP, Zhai YF, Chai WG, Gong ZH, Lu MH (2015) Genome-wide analysis, expression profile of heat shock factor gene family (CaHsfs) and characterisation of CaHsfA2 in pepper (Capsicum annuum L.). BMC Plant Biol 15:151. https://doi.org/10.1186/s12870-015-0512-7
Guo M, Liu JH, Lu JP, Zhai YF, Wang H, Gong ZH, Wang SB, Lu MH (2015) Genome-wide analysis of the CaHsp20 gene family in pepper: comprehensive sequence and expression profile analysis under heat stress. Front Plant Sci 6:806. https://doi.org/10.3389/fpls.2015.00806
Xue GP, Sadat S, Drenth J, McIntyre CL (2014) The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J Exp Bot 65(2):539–557. https://doi.org/10.1093/jxb/ert399
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62(6):670–684. https://doi.org/10.1007/s00018-004-4464-6
Scharf KD, Berberich T, Ebersberger I (1819) Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta 2:104–119. https://doi.org/10.1016/j.bbagrm.2011.10.002
Acknowledgements
This research was supported by the funds from the Natural Science Foundation of Jiangsu Province (BK20180519) and the Priority Academic Program Development of Jiangsu Higher Education Institutions: Modern Horticultural Science (PAPD).
Author information
Authors and Affiliations
Contributions
RT, QY, and XL conceived and designed the research. MH, RT and XL performed ddPCR analysis. RT, SN, GC and MH collected the samples. RT, WZ and SG analyzed the data and performed the bioinformatics analysis. RT and SN determined the starch content of potato tubers. SG detected the reducing sugar content in potato tubers. RT and QY wrote the manuscript. XL and SG edited the English language in this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This research is about transcriptomic analysis in plant (potato). Human participants/animals were not involved in this study.
Informed consent
Not applicable in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing Yang is the first corresponding author and Xiu-Qing Li is the second corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2020_5485_MOESM2_ESM.tif
Supplementary file2 (TIF 88 kb) Expression fold changes of 12 selected genes by RNA sequencing and ddPCR. The value showed the expression changes of DEGs under two different methods, that is DEG expression amount under heat stress/its expression amount under normal condition.
11033_2020_5485_MOESM3_ESM.xlsx
Supplementary file3 (XLSX 32 kb) Gene ontology classification analysis of DEGs between non-stressed potato leaves and heat-stressed potato leaves.
11033_2020_5485_MOESM5_ESM.tif
Supplementary file5 (TIF 119 kb) The starch content (a) and reducing sugar content (b) in potato tubers with different treatments. CK contrast check potato tubers (20 °C day/18 °C night for 3 months),HS heat stressed potato tubers (35 °C day/28 °C night for 3 months). At least three independent biological experiments were conducted to determine the above indicators. The results were presented as mean ± SE. The method of Student’s t test was used for statistical analysis. Megazyme K_AMYL Amylose kit and DNS (3, 5-dinitrosalicylic acid) calorimetry were used to determine the starch content and the reducing sugar content of potato tubers, respectively. The average starch content in dry matter of potato tubers under normal growing conditions was 67.74%, which was significantly higher than that of potato tubers under heat stress (50.09%) (a). However, The results of reducing sugar content in potato tubers was contrary to the trend of starch content. Under normal growing conditions, the average content of reducing sugar content was 11.25 mg/g tuber (fresh weight), which was significantly lower than that of potato tubers grown under high temperature (the average content of reducing sugar was 32.87 mg/g tuber) (b). In general, the decrease of starch content and the increase of total reducing sugar content in potato tubers after heat treatment indicated that a large amount of starch was hydrolyzed into reducing sugar in response to heat stress.
Rights and permissions
About this article
Cite this article
Tang, R., Gupta, S.K., Niu, S. et al. Transcriptome analysis of heat stress response genes in potato leaves. Mol Biol Rep 47, 4311–4321 (2020). https://doi.org/10.1007/s11033-020-05485-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05485-5