Abstract
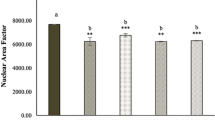
Candida albicans (C. albicans) cell wall beta-glucan has been considered as a potential agent in the treatment of cancers due to its anti-tumor properties. Therefore, in the present study, we investigated the anti-cancer effects of Candida cell wall beta-glucan on Lewis lung carcinoma cell line (LL/2) cells. Beta-glucan of C. albicans cell wall was extracted. LL/2 cell line was cultured, then sphere cells and parental cells were exposed to the different concentrations of beta-glucan extracted from C. albicans (10–6000 μg/ml), for 24, 48 and 72 h. Cytotoxicity of beta-glucan was assayed by MTT test, then RNA extracted from cells population (treated and untreated cells), cDNA synthetized and expression level of Sox2, Oct4, C-myc, Nanog genes were also investigated using Real-time methods. At optimal concentrations of 800 and 1000 μg/ml, the extracted beta-glucan showed a significant cytotoxic effect on both parental and sphere cell populations (p < 0.05). Real-time PCR analysis revealed a decreased expression of Oct4 and Sox2 genes in treatment of cells with beta-glucan compared with control group. Since the extracted beta-glucan showed an inhibitory effect on the expression of Oct4 and Sox2 genes involved in LL/2 metastasis, therefore, beta-glucan can be considered as an anti-tumor agent because of its anti-metastatic properties, however, more in vitro and in vivo studies are needed to provide further evidence on this topic in the future.




Similar content being viewed by others
References
Romo JA, Kumamoto CA (2020) On commensalism of Candida. J Fungi 6(1):16
Kadosh D (2019) Regulatory mechanisms controlling morphology and pathogenesis in Candida albicans. Curr Opin Microbiol 52:27–34
Fesel PH, Zuccaro A (2016) beta-glucan: crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet Biol 90:53–60. https://doi.org/10.1016/j.fgb.2015.12.004
Gow NA, Hube B (2012) Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 15(4):406–412. https://doi.org/10.1016/j.mib.2012.04.005
Vetvicka V, Vetvickova J (2018) Glucans and cancer: comparison of commercially available beta-glucans—Part IV. Anticancer Res 38(3):1327–1333. https://doi.org/10.21873/anticanres.12355
Yin M, Zhang Y, Li H (2019) Advances in research on immunoregulation of macrophages by plant polysaccharides. Front Immunol 10:145
Kim HS, Park KH, Lee HK, Kim JS, Kim YG, Lee JH, Kim KH, Yun J, Hwang BY, Hong JT (2016) Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int Immunopharmacol 39:71–78
Thwe PM, Fritz DI, Snyder JP, Smith PR, Curtis KD, O'Donnell A, Galasso NA, Sepaniac LA, Adamik BJ, Hoyt LR (2019) Syk-dependent glycolytic reprogramming in dendritic cells regulates IL-1β production to β-glucan ligands in a TLR-independent manner. J Leukoc Biol 106(6):1325–1335
Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH (2016) The role of cancer stem cells in recurrent and drug-resistant lung cancer. In: Ahmad A, Gadgeel SM (eds) Lung cancer and personalized medicine: novel therapies and clinical management. Springer, Cham, pp 57–74
Heng WS, Gosens R, Kruyt FA (2019) Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol 160:121–133
Raniszewska A, Polubiec-Kownacka M, Rutkowska E, Domagala-Kulawik J (2019) PD-L1 expression on lung cancer stem cells in metastatic lymph nodes aspirates. Stem Cell Rev Rep 15(2):324–330
Geiger TR, Peeper DS (2009) Metastasis mechanisms. BBA 1796(2):293–308
Das PK, Pillai S, Rakib MA, Khanam JA, Gopalan V, Lam AK, Islam F (2020) Plasticity of cancer stem cell: origin and role in disease progression and therapy resistance. Stem Cell Rev Rep 16:397–412
Roudi R, Mohammadi S, Roudbary M, Mohsenzadegan M (2017) Lung cancer and β-glucans: review of potential therapeutic applications. Invest New Drugs. https://doi.org/10.1007/s10637-017-0449-9
Nassar D, Blanpain C (2016) Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol 11:47–76
Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF (2004) Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 14(1):43–47
Soltanian S, Matin MM (2011) Cancer stem cells and cancer therapy. Tumor Biol 32(3):425–440
Guha D, Banerjee S, Mukherjee S, Dutta A, Das T (2020) Reactive oxygen species: friends or foes of lung cancer? In: Chakraborti S, Parinandi N, Ghosh R, Ganguly N, Chakraborti T (eds) Oxidative stress in lung diseases. Springer, Singapore, pp 331–352
Del Cornò M, Gessani S, Conti L (2020) Shaping the innate immune response by dietary glucans: any role in the control of cancer? Cancers 12(1):155
Geller A, Shrestha R, Yan J (2019) Yeast-derived β-glucan in cancer: novel uses of a traditional therapeutic. Int J Mol Sci 20(15):3618
Bose N, Gorden K, LEONARDO S, GRAFF J, Qiu X, KANGAS T, FRASER KA, JONAS AB, OTTOSON N, Fulton R (2019) Beta-glucan in combination with anti-cancer agents affecting the tumor microenvironment. Google Patents
Sima P, Richter J, Vetvicka V (2019) Glucans as new anticancer agents. Anticancer Res 39(7):3373–3378
Venturella G, Saporita P, Gargano ML (2019) The potential role of medicinal mushrooms in the prevention and treatment of gynecological cancers: a review. Int J Med Mushrooms 21(3):225–235
Baldassano S, Accardi G, Vasto S (2017) Beta-glucans and cancer: the influence of inflammation and gut peptide. Eur J Med Chem 142:486–492
Zhang M, Kim JA, Huang AY-C (2018) Optimizing tumor microenvironment for cancer immunotherapy: β-glucan-based nanoparticles. Front Immunol 9:341
Medina-Gali RM, del Mar O-V, Mercado L, Novoa B, Coll J, Perez L (2018) Beta-glucan enhances the response to SVCV infection in zebrafish. Dev Comp Immunol 84:307–314
Sun X, Gao Y, Ding Z, Zhao Y, Yang Y, Sun Q, Yang X, Ge W, Xu X, Cheng R (2020) Soluble beta-glucan salecan improves vaginal infection of Candida albicans in mice. Int J Biol Macromol 148:1053–1060
Nassar SA, Mohamed AM, Sedky D, El-Shemy A, Allam AM (2018) Oral and intraperitoneal administration of β-glucan and its immunomodulatory effect against staphylococcus aureus infection in rats. Int J Pharm Phytopharmacol Res 8(2):1–7
Saravanakumar K, Jeevithan E, Hu X, Chelliah R, Oh D-H, Wang M-H (2020) Enhanced anti-lung carcinoma and anti-biofilm activity of fungal molecules mediated biogenic zinc oxide nanoparticles conjugated with β-D-glucan from barley. J Photochem Photobiol, B 203:111728
Lee JN, Lee DY, Ji IH, Kim GE, Kim HN, Sohn J, Kim S, Kim CW (2001) Purification of soluble beta-glucan with immune-enhancing activity from the cell wall of yeast. Biosci Biotechnol Biochem 65(4):837–841. https://doi.org/10.1271/bbb.65.837
Nasrollahi Z, Roudbar Mohammadi S, Atyabi F, Zuhair Sarraf H, Yadegari MH, Esfandyari M, Mollarazi E (2013) A rapid method for extraction of water soluble β(1,3) glucan from the cell wall of Candida albicans. Pathobiol Res 16(1):89–97
Kim YT, Kim EH, Cheong C, Williams DL, Kim CW, Lim ST (2000) Structural characterization of beta-D-(1 –%3e 3, 1 –%3e 6)-linked glucans using NMR spectroscopy. Carbohydr Res 328(3):331–341. https://doi.org/10.1016/s0008-6215(00)00105-1
Patel S, Gheewala N, Suthar A, Shah A, Patel S (2008) In-Vitro cytotoxicity activity of Solanum Nigrum extract against Hela cell line and Vero cell line. Int J Pharm Pharm Sci 1:38–46
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1(5):2315–2319. https://doi.org/10.1038/nprot.2006.339
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236(1–2):52–60
Albeituni SH, Ding C, Liu M, Hu X, Luo F, Kloecker G, Bousamra M 2nd, Zhang HG, Yan J (2016) Yeast-derived particulate beta-glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol 196(5):2167–2180. https://doi.org/10.4049/jimmunol.1501853
Shackleton M, Quintana E, Fearon ER, Morrison SJ (2009) Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 138(5):822–829. https://doi.org/10.1016/j.cell.2009.08.017
Abugomaa A, Elbadawy M, Yamawaki H, Usui T, Sasaki K (2020) Emerging roles of cancer stem cells in bladder cancer progression, tumorigenesis, and resistance to chemotherapy: a potential therapeutic target for bladder cancer. Cells 9(1):235
Takahashi K, Asano N, Imatani A, Kondo Y, Saito M, Takeuchi A, Jin X, Saito M, Hatta W, Asanuma K (2020) Sox2 induces tumorigenesis and angiogenesis of early stage esophagealsquamous cell carcinoma through secretion of Suprabasin. Carcinogenesis. https://doi.org/10.1093/carcin/bgaa014
Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K (2014) Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 9(1):e84941. https://doi.org/10.1371/journal.pone.0084941
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J, Chen R, Zhou Y (2013) Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int J Oncol 42(2):453–459. https://doi.org/10.3892/ijo.2012.1720
Darini CY, Pisani DF, Hofman P, Pedeutour F, Sudaka I, Chomienne C, Dani C, Ladoux A (2012) Self-renewal gene tracking to identify tumour-initiating cells associated with metastatic potential. Oncogene 31(19):2438–2449. https://doi.org/10.1038/onc.2011.421
Xiang R, Liao D, Cheng T, Zhou H, Shi Q, Chuang TS, Markowitz D, Reisfeld RA, Luo Y (2011) Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer 104(9):1410–1417. https://doi.org/10.1038/bjc.2011.94
Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X (2013) The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal 25(5):1264–1271. https://doi.org/10.1016/j.cellsig.2013.02.013
Villodre ES, Kipper FC, Pereira MB, Lenz G (2016) Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev 51:1–9. https://doi.org/10.1016/j.ctrv.2016.10.003
Yoon TJ, Kim TJ, Lee H, Shin KS, Yun YP, Moon WK, Kim DW, Lee KH (2008) Anti-tumor metastatic activity of β-glucan purified from mutated Saccharomyces cerevisiae. Int Immunopharmacol 8(1):36–42
Queiroz EA, Fortes ZB, da Cunha MA, Barbosa AM, Khaper N, Dekker RF (2015) Antiproliferative and pro-apoptotic effects of three fungal exocellular β-glucans in MCF-7 breast cancer cells is mediated by oxidative stress, AMP-activated protein kinase (AMPK) and the Forkhead transcription factor, FOXO3a. Int J Biochem Cell Biol 67:14–24
Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N (2005) Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J Cancer Res Clin Oncol 131(8):527–538
Funding
This study was financially supported by Iran University of Medical Sciences with Grant Number (30-29412)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors declare no conflict of interest.
Ethical approval:
This study was approved by the research committee of Iran University of Medical Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadeghi, F., Peymaeei, F., Falahati, M. et al. The effect of Candida cell wall beta-glucan on treatment-resistant LL/2 cancer cell line: in vitro evaluation. Mol Biol Rep 47, 3653–3661 (2020). https://doi.org/10.1007/s11033-020-05459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05459-7