Abstract
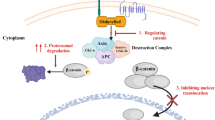
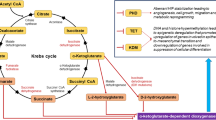
In recent years, sulforaphane (SFN) has been shown to have antitumor effects. To better understand the molecular basis of SFN intervention in estrogen-dependent breast cancer, integrated multi-omics data analysis was used to provide evidence and insights into molecular biology. MCF-7 breast cancer cells were treated with estradiol (E2) or/and SFN. Genome-wide DNA methylation analysis was performed by using microarray platforms. The protein profile was analyzed by TMT labeled HPLC-MS/MS. The metabolic profile was obtained by GC–MS and UPLC–MS methods. Multivariate statistics analyses, such as PCA and hierarchical clustering, were performed. The Gene Ontology (GO) and KEGG analysis were used to perform enrichment analysis of biological processes and pathways. A set of differentially methylated genes and differentially expressed proteins and metabolites were found, which indicated that SFN may reverse the adverse effects induced by E2. Integrated analysis revealed cancer genes that responded to estrogen and other pathways frequently associated with cancer. Co-pathway analysis revealed that the reversal effects of SFN were associated with purine metabolism and glutathione metabolism. The integrated omics analysis outlined a promising blueprint of the relationship of biological molecules in different dimensions, which will be beneficial for understanding the mechanism of anti-breast cancer effects and for new targets of medicines.





Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Yage JD (2000) Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr 27:67–73
Yager JD (2015) Mechanisms of estrogen carcinogenesis: the role of E2/E1-quinone metabolites suggests new approaches to preventive intervention—a review. Steroids 99:56–60
Cavalieri EL, Rogan EG (2011) Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol 125:169–180
Yang YM, Sun D, Kandhi S, Froogh G, Zhuge J, Huang W, Hammock BD, Huang A (2018) Estrogen-dependent epigenetic regulation of soluble epoxide hydrolase via DNA methylation. Proc Natl Acad Sci U S A 115(3):613–618
Mandal S, Khan P, Lin L, Davie JR (2011) Metabolomics and transcriptional responses in estrogen receptor positive breast cancer cells. Breast Cancer - Carcinog, Cell Growth Signal Pathw. https://doi.org/10.5772/22987
Yang L, Palliyaguru DL, Kensler TW (2016) Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin Oncol 43:146–153
Dong QQ, Wang QT, Wang L, Jiang YX, Liu ML, Hu HJ, Liu Y, Zhou H, He HP, Zhang TC, Luo XG (2018) SMYD3-associated pathway is involved in the anti-tumor effects of sulforaphane on gastric carcinoma cells. Food Sci Biotechnol 27:1165–1173
Khaleel SA, Raslan NA, Alzokaky AA, Ewees MG, Ashour AA, Abdel-Hamied HE, Abd-Allah AR (2019) Contrast media (meglumine diatrizoate) aggravates renal inflammation, oxidative DNA damage and apoptosis in diabetic rats which is restored by sulforaphane through Nrf2/HO-1 reactivation. Chem Biol Interact 309:108689
Subedi L, Lee JH, Yumnam S, Ji E, Kim SY (2019) Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-kappaB inhibition and Nrf2/HO-1 activation. Cells 8(2):E194
Russo M, Spagnuolo C, Russo GL, Skalicka-Wozniak K, Daglia M, Sobarzo-Sanchez E, Nabavi SF, Nabavi SM (2018) Nrf2 targeting by sulforaphane: a potential therapy for cancer treatment. Crit Rev Food Sci Nutr 58:1391–1405
Paul B, Li Y, Tollefsbol TO (2018) The effects of combinatorial genistein and sulforaphane in breast tumor inhibition: role in epigenetic regulation. Int J Mol Sci 19(6):E1754
Li Y, Buckhaults P, Li S, Tollefsbol T (2018) Temporal efficacy of a sulforaphane-based broccoli sprout diet in prevention of breast cancer through modulation of epigenetic mechanisms. Cancer Prev Res (Phila) 11:451–464
Royston KJ, Udayakumar N, Lewis K, Tollefsbol TO (2017) A novel combination of withaferin a and sulforaphane inhibits epigenetic machinery, cellular viability and induces apoptosis of breast cancer cells. Int J Mol Sci 18(5):E1092
Lecuyer L, Victor Bala A, Deschasaux M, Bouchemal N, Nawfal Triba M, Vasson MP, Rossary A, Demidem A, Galan P, Hercberg S, Partula V, Le Moyec L, Srour B, Fiolet T, Latino-Martel P, Kesse-Guyot E, Savarin P, Touvier M (2018) NMR metabolomic signatures reveal predictive plasma metabolites associated with long-term risk of developing breast cancer. Int J Epidemiol 47:484–494
Hadi NI, Jamal Q (2015) "OMIC" tumor markers for breast cancer: a review. Pak J Med Sci 31:1256–1262
Cao S, Wang L, Zhang Z, Chen F, Wu Q, Li L (2018) Sulforaphane-induced metabolomic responses with epigenetic changes in estrogen receptor positive breast cancer cells. FEBS Open Bio 8:2022–2034
Li C, Tang L, Zhao L, Li L, Xiao Q, Luo X, Peng W, Ren G, Tao Q, Xiang T (2015) OPCML is frequently methylated in human colorectal cancer and its restored expression reverses EMT via downregulation of smad signaling. Am J Cancer Res 5:1635–1648
Zhou F, Tao G, Chen X, Xie W, Liu M, Cao X (2014) Methylation of OPCML promoter in ovarian cancer tissues predicts poor patient survival. Clin Chem Lab Med 52:735–742
Duarte-Pereira S, Paiva F, Costa VL, Ramalho-Carvalho J, Savva-Bordalo J, Rodrigues A, Ribeiro FR, Silva VM, Oliveira J, Henrique R, Jeronimo C (2011) Prognostic value of opioid binding protein/cell adhesion molecule-like promoter methylation in bladder carcinoma. Eur J Cancer 47:1106–1114
Liang CY, Wang LJ, Chen CP, Chen LF, Chen YH, Chen H (2010) GCM1 regulation of the expression of syncytin 2 and its cognate receptor MFSD2A in human placenta. Biol Reprod 83:387–395
Rowland BD, Bernards R, Peeper DS (2005) The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol 7:1074–1082
Hu D, Gur M, Zhou Z, Gamper A, Hung MC, Fujita N, Lan L, Bahar I, Wan Y (2015) Interplay between arginine methylation and ubiquitylation regulates KLF4-mediated genome stability and carcinogenesis. Nat Commun 6:8419
Fleischer T, Edvardsen H, Solvang HK, Daviaud C, Naume B, Borresen-Dale AL, Kristensen VN, Tost J (2014) Integrated analysis of high-resolution DNA methylation profiles, gene expression, germline genotypes and clinical end points in breast cancer patients. Int J Cancer 134:2615–2625
Prest SJ, May FE, Westley BR (2002) The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J 16:592–594
Lin CY, Strom A, Vega VB, Kong SL, Yeo AL, Thomsen JS, Chan WC, Doray B, Bangarusamy DK, Ramasamy A, Vergara LA, Tang S, Chong A, Bajic VB, Miller LD, Gustafsson JA, Liu ET (2004) Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol 5(9):R66
Huang SH, Hsu MH, Hsu SC, Yang JS, Huang WW, Huang AC, Hsiao YP, Yu CC, Chung JG (2013) Phenethyl isothiocyanate triggers apoptosis in human malignant melanoma A375S2 cells through reactive oxygen species and the mitochondria-dependent pathways. Hum Exp Toxicol 33(3):270–283
Jin GZ, Zhang Y, Cong WM, Wu X, Wang X, Wu S, Wang S, Zhou W, Yuan S, Gao H, Yu G, Yang W (2018) Phosphoglucomutase 1 inhibits hepatocellular carcinoma progression by regulating glucose trafficking. PLoS Biol 16:e2006483
Azevedo MF, Faucz FR, Bimpaki E, Horvath A, Levy I, de Alexandre RB, Ahmad F, Manganiello V, Stratakis CA (2014) Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev 35:195–233
Abena SA, Raghothama C, Patrick GS, Nancy ED, Kala V, Akhilesh P, Thomas WK (2012) Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat 132(1):175–187
Wu Q, Odwin-Dacosta S, Cao S, Yager JD, Tang WY (2019) Estrogen down regulates COMT transcription via promoter DNA methylation in human breast cancer cells. Toxicol Appl Pharmacol 367:12–22
Yager JD, Davidson NE (2006) Estrogen carcinogenesis in breast cancer. N Engl J Med 354(3):270–282
Haracska L, Yu SL, Johnson RE, Prakash L, Prakash S (2000) Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase eta. Nat Genet 25:458–461
Rocha JC, Busatto FF, Guecheva TN, Saffi J (2016) Role of nucleotide excision repair proteins in response to DNA damage induced by topoisomerase II inhibitors. Mutat Res Rev Mutat Res 768:68–77
Riso P1, Martini D, Møller P, Loft S, Bonacina G, Moro M, Porrini M (2010) DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis 25(6):595–602
Hać A, Brokowska J, Rintz E, Bartkowski M, Węgrzyn G, Herman-Antosiewicz A (2019) Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur J Nutr. https://doi.org/10.1007/s00394-019-01995-6
Zhu HY, Luo H, Zhang WW, Shen ZJ, Hu X, Zhu XQ (2016) Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther 10:1885–1895
Makovec T (2019) Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol 53:148–158
Estrela JM, Ortega A, Obrador E (2006) Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 43(2):143–181
Nicola T, Roberta R, Mariapaola N, Barbara M, Anna LF, Maria AP, Umberto MM, Cinzia D (2013) Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013:972913
Xu Y, Han X, Li Y, Min H, Zhao X, Zhang Y, Qi Y, Shi J, Qi S, Bao Y, Nie G (2019) Sulforaphane mediates glutathione depletion via polymeric nanoparticles to restore cisplatin chemosensitivity. ACS Nano 13(11):13445–13455
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Nos. 81903365, 81530088 and 81728018), Natural Science Foundation of Jiangsu Province (No. BK20161571), Natural Science Foundation of the Jiangsu Higher Education Institution of China (16KJA330002), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Top-notch Academic Programs Project of Jiangsu Higher Education Institution, (TAPP,PPZY2015A067).The funders had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: QW and FC. Performed the experiments: SYC. Analyzed the data: HH, YYW and ZZ. Contributed reagents/materials/analyses tools: LL and FC. Wrote the paper: SYC, QW. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, H., Cao, S., Zhang, Z. et al. Multiple omics analysis of the protective effects of SFN on estrogen-dependent breast cancer cells. Mol Biol Rep 47, 3331–3346 (2020). https://doi.org/10.1007/s11033-020-05403-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05403-9