Abstract
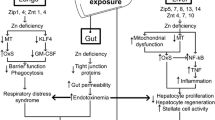
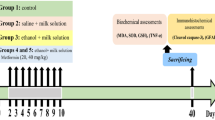
Recently published experimental and clinical studies indicate that oxidative stress leads to the pathogenesis and progression of alcohol-induced tissue injuries. Quercetin is a type of flavonoid compound that influences antioxidant and anti-inflammatory activities have protective and therapeutic effects for treating various diseases including diabetes mellitus and neuro-degenerative diseases. In this study, fetal alcohol syndrome was tested in rat models, with the aim of verifying the protective effect of quercetin in preventing alcohol-induced liver and lymphoid tissue (thymus, spleen, and lymph nodes) injuries on the 21st day for the offspring of alcohol treated mother rats. The pregnant rats were randomly assigned into four groups. The control group (C) (n = 3) of pregnant rats received only physiological saline intraperitoneally (i.p.) throughout the pregnancy (1 to 21 days gestation) and during lactation until postnatal day 21. The quercetin positive control group (QT) of pregnant rats (n = 3) received quercetin at 50 mg/kg/days i.p. for the same period. The ethanol treatment group (E) (n = 3) of pregnant rats received 1 ml/day of 40% v/v ethanol (4 g/kg) intragastrically (i.g) for the same period. The model group of pregnant rats (EQ) received ethanol + quercetin (n = 3) with a dose of 1 ml/day of v/v ethanol (4 g/kg i.g.) and quercetin at 50 mg/kg body weight per day i.p. for the same period. Ten offspring were used in each of the C, QT, E and EQ groups. Malondialdehyde (MDA), protein carbonyl content (PC) and chemiluminescence levels (CL) in liver and lymphoid tissues significantly increased in group E versus the C group (P < 0.05–P < 0.001) whereas glutathione levels (GSH), glutathione reductase (GR), glutathione peroxidase (GP), superoxide dismutase (SOD), and catalase (CAT) activities significantly decreased in group E compared to the C group (P < 0.05–< 0.001). However, tissue MDA, PC, and CL levels decreased in the EQ group compared to group E. GSH level, GP, GR, SOD, and CAT activity were significantly increased by quercetin (P < 0.05–P < 0.001). The plasma TNFα, IL-1β, and IL-6 levels and NF-κB activation significantly increased in group E compared to the C and QT groups, but IL-10 significantly decreased in group E compared to the C and QT groups. The TNFα, IL-1β, and IL-6 levels and NF-κB activation significantly decreased in group EQ compared to group E. In conclusion, quercetin has a protective effect against maternal alcohol-induced oxidative and inflammatory damage in the liver and lymphoid tissues of newborn rats.




Similar content being viewed by others
Abbreviations
- FASD:
-
Fetal alcohol spectrum disorder
- ROS:
-
Reactive oxygen species
- LP:
-
Lipid peroxidation
- PC:
-
Protein carbonyl content
- CL:
-
Chemiluminescence assay
- GSH:
-
Reduced glutathione
- GP:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa B
- PIC:
-
Proinflammatory cytokine
References
Akhtar F, Rouse CA, Catano G, Montalvo M et al (2017) Acute maternal oxidant exposure causes susceptibility of the fetal brain to inflammation and oxidative stress. J Neuroinflammation 14:195
Goodlett CR, Horn KH (2001) Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health 25(3):175–184
Williams JF, Smith VC (2015) Committee on substance abuse a fetal alcohol spectrum disorders. Pediatrics 136(5):e1395
Barr HM, Streissguth AP (2001) Identifying maternal self-reported alcohol use associated with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 25:283–287
Marino MD, Aksenov MY, Kelly SJ (2004) Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int J Dev Neurosci 22:363–377
Amini SA, Dunstan H, Raymond P, Murdoch PDR (1996) Oxidative stress and the fetotoxicity of alcohol consumption during pregnancy. Free Radical Biol Med 21(3):357–365. https://doi.org/10.1016/0891-5849(96)00027-5
Aydın B (2011) Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats. Pestic Biochem Physiol 100:65–171
Sibley D, Jerrells TR (2000) Alcohol consumption by C57BL/6 mice is associated with depletion of lymphoid cells from the Gut-associated lymphoid tissues and altered resistance to oral infections with Salmonella typhimuriu. J Infect Dis 182:482–489
Walia AS, Pruitt KM, Rodgers JD, Lamon EW (1987) In vitro effect of ethanol on cell-mediated cytotoxicity by murine spleen cells. Immunopharmacology 13(1):11–210
İnce E, Curabeyoğlu F, Akyol S (2019) Oxidative stress in lymphoid tissues and complement activation in alcoholic mother rats and their newborns. General Biophys Physiol 38(1):91–100
Bodnar T, Hill LA, Weinberg J (2016) Evidence for an immune signature of prenatal alcohol exposure in female rats. Brain Behav Immun 58:130–141
Zhu M, Zhou X, Zhao J (2017) Quercetin prevents alcohol-induced liver injury through targeting of PI3 K/Akt/Nuclear Factor-κB and STAT3 signaling pathway. Exp Ther Med 14:6169–6175
Li X, Jin Q, Yao Q, Xu B, Li L et al (2018) The Flavonoid Quercetin ameliorates liver inflammation and fibrosis by regulating hepatic macrophages activation and polarization in mice. Front Pharmacol 9:72. https://doi.org/10.3389/fphar.2018.00072
Vanhees K, Godschalk RW, Sanders A et al (2011) Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology 290(2–3):350–358
Romaszko E, Wiczkowski W, Romaszko J et al (2014) Exposure of breastfed infants to quercetin after consumption of a single meal rich in quercetin by their mothers. Mol Nutr Food Res 58(2):221–228
Lundquist F (1959) The determination of ethyl alcohol in blood and tissues. In: Glick D (ed) Methods of biochemical analysis. Interscience Publishers Inc, New York, p 217
Singh RP, Padmavathi B, Rao R (2000) Modulatory influence of Adhatoda vesicaleaf extract on the enzymes of xenobiotic metabolism, antioxidant status and lipid peroxidation in mice. Mol Cell Biochem 213:9–109
Levine RL, Williams JA, Stadtman ER et al (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Davies GR, Simmonds NJ, Stevens TRJ et al (1992) Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut 33:1467–1472
Ohara Y, Peterson TE, Harrison DG (1993) Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91:2546–2551
Haklar G, Yüksel M, Yalçın AS (1998) Chemiluminescence in the measurement of free radicals: theory and application on a tissue injury model. Marmara Med. J 11:56–60
Carlberg I, Mannervik B (1981) Purification and characterization of glutathione reductase from calf liver glutathione reductase assays. Methods Enzymol 113:484–495
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Carlberg I, Mannervik B (1981) Purification and characterization of glutathione reductase from calf liver glutathione reductase assays. Methods Enzymol 113:484–495
Sun Y, Oberley LW, Li Y (1998) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Aebi H (1985) Catalase. Methods Enzymol
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Molina PE, Hoek JB, Nelson S, Guidot DM, Lang CH et al (2003) Mechanisms of alcohol-induced tissue injury. Alcohol Clin Exp Res 27:563–575
Wu D, Cederbaum AI (2003) Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 27(4):277–284
Wu CH, Chen CC, Lai CY, Hung TH, Lin CC et al (2016) Treatment with TO901317, a synthetic liver X receptor agonist, reduces brain damage and attenuates neuroinflammation in experimental intracerebral hemorrhage. J Neuroinflammation 13:62
Kahraman A, Cakar H, Koken T (2012) The protective effect of quercetin on long-term alcohol consumption-induced oxidative stress. Mol Biol Rep 39:2789–2794
Ambade A, Mandrekar P (2012) Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol. https://doi.org/10.1155/2012/853175
Coll TA, Chaufan G, Pérez-Tito LG et al (2018) Cellular and molecular oxidative stress-related effects in uterine myometrial and trophoblast-decidual tissues after perigestational alcohol intake up to early mouse organogenesis. Mol Cell Biochem 440(1–2):89–104
Movileanu L, Neagoe I, Flonta ML (2000) Interaction of the antioxidant flavonoid quercetin with planar lipid bilayers. Int J Pharm 205(1–2):135–146
Vanhees K, Godschalk RW, Sanders A et al (2011) Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology 290(2–3):350–358
Afasenev IB, Dcrozhko A, Brodski AV et al (1989) Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Biopharmacol 38(11):1763–1769
Cederbaum AI, Lu Y, Wu D (2009) Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83:519–548
Ding RB, Tian K, Cao YW et al (2015) Protective effect of panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J Agric Food Chem 63:2413–2422
Choi EJ, Chee KM, Lee BH (2003) Anti- and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol 482(1–3):281–285
Tong M, Longato L, Ramirez T et al (2014) Therapeutic reversal of chronic alcohol-related steatohepatitis with the ceramide inhibitor myriocin. Int J Exp Pathol 95(1):49–63
Chen L, Deng H, Cui H, Fang J, Zuo Z et al (2018) Inflammatory responses and inflammation associated diseases in organs. Oncotarget 9(6):7204–7218
Burd L, Blair J, Dropps K (2012) Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J Perinatol 32:652–659
Tan Z, Luo M, Yang J et al (2016) Chlorogenic acid inhibits cholestatic liver injury induced by α-naphthylisothiocyanate: involvement of STAT3 and NFκB signalling regulation. J Pharm Pharmacol 68:1203–1213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no financial or any other conflict of interest in this manuscript with third parties. The author is responsible for the content and writing of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ince, E. The protective effect of quercetin in the alcohol-induced liver and lymphoid tissue injuries in newborns. Mol Biol Rep 47, 451–459 (2020). https://doi.org/10.1007/s11033-019-05148-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05148-0