Abstract
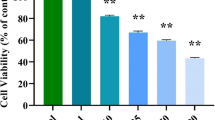
Plumbagin (PLB) is a phytochemical being used for centuries in traditional medicines. Recently, its capacity to inhibit the development of human tumors has been observed, through the induction of apoptosis, cell cycle arrest, and inhibition of angiogenesis and metastasis. Here we evaluated the mechanism of action of PLB in the kidney adenocarcinoma 786-O cell line, which are metabolizing cells important for toxicology studies. After the treatment with PLB, we observed increased apoptosis and cell cycle arrest in S and G2/M phases, starting at 5 µM. In addition, PLB was cytotoxic, genotoxic and induced loss of cell membrane integrity. Regarding gene expression, treatment with 7.5 µM PLB reduced the amount of MTOR, BCL2 and ATM transcripts, and increased CDKN1A (p21) transcripts. Phosphorylation levels of yH2AX was increased and MDM2 protein level was reduced following the treatment with PLB, demonstrating its genotoxic effect. Our results suggest that PLB acts in molecular pathways related to the control of proliferation and cell death in 786-O cells.






Similar content being viewed by others
References
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661. https://doi.org/10.1021/acs.jnatprod.5b01055
Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R (2009) Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci 5(1):1–11
Greenwell M, Rahman PK (2015) Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res 6(10):4103–4112. https://doi.org/10.13040/IJPSR.0975-8232.6(10).4103-12
Gillet JP, Varma S, Gottesman MM (2013) The clinical relevance of cancer cell lines. J Natl Cancer Inst 105(7):452–458. https://doi.org/10.1093/jnci/djt007
Guengerich FP (2001) Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol 14(6):611–650
Tandon VK, Kumar S (2013) Recent development on naphthoquinone derivatives and their therapeutic applications as anticancer agents. Expert Opin Ther Pat 23(9):1087–1108. https://doi.org/10.1517/13543776.2013.798303
Roy A, Bharadvaja N (2018) Biotechnological approaches for the production of pharmaceutically important compound: plumbagin. Curr Pharm Biotechnol 19(5):372–381. https://doi.org/10.2174/1389201019666180629143842
Acharya BR, Bhattacharyya B, Chakrabarti G (2008) The natural naphthoquinone plumbagin exhibits antiproliferative activity and disrupts the microtubule network through tubulin binding. Biochemistry 47(30):7838–7845. https://doi.org/10.1021/bi800730q
Lee JH, Yeon JH, Kim H, Roh W, Chae J, Park HO, Kim DM (2012) The natural anticancer agent plumbagin induces potent cytotoxicity in MCF-7 human breast cancer cells by inhibiting a PI-5 kinase for ROS generation. PLoS ONE 7(9):e45023. https://doi.org/10.1371/journal.pone.0045023
Kang CG, Im E, Lee HJ, Lee EO (2017) Plumbagin reduces osteopontin-induced invasion through inhibiting the Rho-associated kinase signaling pathway in A549 cells and suppresses osteopontin-induced lung metastasis in BalB/c mice. Bioorg Med Chem Lett 27(9):1914–1918. https://doi.org/10.1016/j.bmcl.2017.03.047
Zhou ZW, Li XX, He ZX, Pan ST, Yang Y, Zhang X, Chow K, Yang T, Qiu JX, Zhou Q, Tan J, Wang D, Zhou SF (2015) Induction of apoptosis and autophagy via sirtuin1- and PI3 K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells. Drug Des Dev Ther 9:1511–1554. https://doi.org/10.2147/DDDT.S75976
Zhang J, Peng S, Li X, Liu R, Han X, Fang J (2017) Targeting thioredoxin reductase by plumbagin contributes to inducing apoptosis of HL-60 cells. Arch Biochem Biophys 619:16–26. https://doi.org/10.1016/j.abb.2017.02.007
Raghu D, Karunagaran D (2014) Plumbagin downregulates Wnt signaling independent of p53 in human colorectal cancer cells. J Nat Prod 77(5):1130–1134. https://doi.org/10.1021/np4010085
Aziz MH, Dreckschmidt NE, Verma AK (2008) Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res 68(21):9024–9032. https://doi.org/10.1158/0008-5472.CAN-08-2494
Baig S, Seevasant I, Mohamad J, Mukheem A, Huri HZ, Kamarul T (2016) Potential of apoptotic pathway-targeted cancer therapeutic research: where do we stand? Cell Death Dis 7:e2058. https://doi.org/10.1038/cddis.2015.275
Bai J, Li Y, Zhang G (2017) Cell cycle regulation and anticancer drug discovery. Cancer Biol Med 14(4):348–362. https://doi.org/10.20892/j.issn.2095-3941.2017.0033
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Boyd JM, Huang L, Xie L, Moe B, Gabos S, Li XF (2008) A cell-microelectronic sensing technique for profiling cytotoxicity of chemicals. Anal Chim Acta 615(1):80–87. https://doi.org/10.1016/j.aca.2008.03.047
Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R (2008) The comet assay: topical issues. Mutagenesis 23(3):143–151. https://doi.org/10.1093/mutage/gem051
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):e36
Knights KM, Rowland A, Miners JO (2013) Renal drug metabolism in humans: the potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT). Br J Clin Pharmacol 76(4):587–602. https://doi.org/10.1111/bcp.12086
Sumsakul W, Chaijaroenkul W, Na-Bangchang K (2015) In vitro inhibitory effects of plumbagin, the promising antimalarial candidate, on human cytochrome P450 enzymes. Asian Pac J Trop Med 8(11):914–918. https://doi.org/10.1016/j.apjtm.2015.10.016
Ono T, Ota A, Ito K, Nakaoka T, Karnan S, Konishi H, Furuhashi A, Hayashi T, Yamada Y, Hosokawa Y, Kazaoka Y (2015) Plumbagin suppresses tumor cell growth in oral squamous cell carcinoma cell lines. Oral Dis 21(4):501–511. https://doi.org/10.1111/odi.12310
Khaw AK, Sameni S, Venkatesan S, Kalthur G, Hande MP (2015) Plumbagin alters telomere dynamics, induces DNA damage and cell death in human brain tumour cells. Mutat Res, Genet Toxicol Environ Mutagen 793:86–95. https://doi.org/10.1016/j.mrgentox.2015.06.004
Somasundaram V, Hemalatha SK, Pal K, Sinha S, Nair AS, Mukhopadhyay D, Srinivas P (2016) Selective mode of action of plumbagin through BRCA1 deficient breast cancer stem cells. BMC Cancer 16:336. https://doi.org/10.1186/s12885-016-2372-4
Yang J, Xu ZP, Huang Y, Hamrick HE, Duerksen-Hughes PJ, Yu YN (2004) ATM and ATR: sensing DNA damage. World J Gastroenterol 10(2):155–160
Khalil H, Deeni Y (2015) NRF2 inhibition causes repression of ATM and ATR expression leading to aberrant DNA damage response. BioDiscovery 15(1):e8964
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276(45):42462–42467. https://doi.org/10.1074/jbc.C100466200
Eischen CM (2011) Decreased Mdm2 levels after DNA damage: antibody masking or protein degradation? Cell Cycle 10(9):1347. https://doi.org/10.4161/cc.10.9.15437
Stommel JM, Wahl GM (2004) Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J 23(7):1547–1556. https://doi.org/10.1038/sj.emboj.7600145
Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS, Bhat KP, Godfrey VL, Evan GI, Zhang Y (2007) Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell 12(4):355–366. https://doi.org/10.1016/j.ccr.2007.09.007
Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, Shaik S, Wan L, Ang XL, Mock C, Yin H, Stommel JM, Gygi S, Lahav G, Asara J, Xiao ZX, Kaelin WG Jr, Harper JW, Wei W (2010) Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell 18(2):147–159. https://doi.org/10.1016/j.ccr.2010.06.015
Vega F, Medeiros LJ, Leventaki V, Atwell C, Cho-Vega JH, Tian L, Claret FX, Rassidakis GZ (2006) Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res 66(13):6589–6597. https://doi.org/10.1158/0008-5472.CAN-05-3018
Fu C, Gong Y, Shi X, Sun Z, Niu M, Sang W, Xu L, Zhu F, Wang Y, Xu K (2016) Plumbagin reduces chronic lymphocytic leukemia cell survival by downregulation of Bcl-2 but upregulation of the Bax protein level. Oncol Rep 36(3):1605–1611. https://doi.org/10.3892/or.2016.4950
Ahmad A, Banerjee S, Wang Z, Kong D, Sarkar FH (2008) Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J Cell Biochem 105(6):1461–1471. https://doi.org/10.1002/jcb.21966
Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E (2010) Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res 704(1–3):12–20. https://doi.org/10.1016/j.mrrev.2010.01.009
Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282(5393):1497–1501
Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL (2008) Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett 259(1):82–98
Hsu YL, Cho CY, Kuo PL, Huang YT, Lin CC (2006) Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. J Pharmacol Exp Ther 318(2):484–494. https://doi.org/10.1124/jpet.105.098863
Kuo PL, Hsu YL, Cho CY (2006) Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther 5(12):3209–3221. https://doi.org/10.1158/1535-7163.MCT-06-0478
Tian L, Yin D, Ren Y, Gong C, Chen A, Guo FJ (2012) Plumbagin induces apoptosis via the p53 pathway and generation of reactive oxygen species in human osteosarcoma cells. Mol Med Rep 5(1):126–132. https://doi.org/10.3892/mmr.2011.624
Klotz LO, Hou X, Jacob C (2014) 1,4-naphthoquinones: from oxidative damage to cellular and inter-cellular signaling. Molecules 19(9):14902–14918. https://doi.org/10.3390/molecules190914902
Acknowledgements
This study was supported by CNPq, CAPES, Fundação Araucária and FINEP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mancilla, I.A., Coatti, G.C., Biazi, B.I. et al. Molecular pathways related to the control of proliferation and cell death in 786-O cells treated with plumbagin. Mol Biol Rep 46, 6071–6078 (2019). https://doi.org/10.1007/s11033-019-05042-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05042-9