Abstract
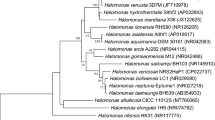
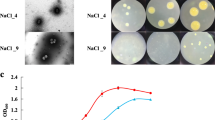
The study aims to find out osmoadaptive mechanism used to overcome the salinity stress by Halomonas sp SBS 10 isolated from the saltern crystallizer ponds of the Sambhar Salt Lake and its taxonomic position using neighbor-joining algorithm. The strain SBS 10 was tested for accumulation of two major compatable solutes betaine and ectoine and was observed that osmoprotection in the strain SBS 10 is achieved by the accumulation of betaine or by the de-novo synthesis of betaine or ectoine. Amount of endogenous content of the betaine and ectoine per milligram of cell biomass was estimated to be 581 µg, 587 µg, 588 µg, 617 µg, and 761 µg for betaine and 1.52 µg, 2.74 µg, 3.14 µg, 3.50 µg, and 52.67 µg for ectoine, when exposed to 5, 10, 15, 20 and 25% of NaCl concentration. Results obtained from HPLC analysis showed that the betaine accumulation suppresses the de-novo synthesis of ectoine partially at low NaCl concentration in the growth medium. However, at a high NaCl concentration, the ectoine concentration increases abruptly as compared to the betaine. This indicates that the ectoine accumulation is transcriptionally up-regulated by the salinity stress. Phylogenetic analysis based on the neighbor-joining algorithm included the strain SBS 10 in the genus Halomonas of the family Halomonadaceae belonging to the class Gammaproteobacteria. Most closely related type strain was found to be Halomonas gudaonensis SL014B-69T (98.2% similarity). Ultrastructure characteristics showed the strain to be non-spore forming rod, 0.3–0.4 × 0.75–1.65 μm in size and motile with the help of peritrichous flagella.




Similar content being viewed by others
References
Haba RR, Arahal DR, Sánchez-Porro C, Ventosa A (2014) The family halomonadaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes. Springer, Berlin, Heidelberg
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Bowers KJ, Mesbah NM, Wiegel J (2009) Biodiversity of polyextremophilic bacteria: does combining the extremes of high salt, alkaline pH and elevated temperature approach a physico-chemical boundary for life? Saline Syst 5:9
Bowers KJ, Wiegel J (2011) Temperature and pH optima of extremely halophilic Archaea: a mini-review. Extremophiles 15:119–128
Oueriaghli N, Gonza´lez-Domenech CM, Martı´nez-Checa F, Muyzer G, Ventosa A, Quesada E, Be´jar V (2014) Diversity and distribution of Halomonas in RamblaSalada, a hypersaline environment in the southeast of Spain. FEMS Microbiol Ecol 87:460–474
Jadhav K, Kushwah B, Jadhav I (2018) Insight into compatible solutes from halophiles: exploring significant applications in biotechnology. In: Singh J, Sharma D, Kumar G, Sharma N (eds) Microbial bioprospecting for sustainable development. Springer, Singapore
Knapp S, Landstein R, Galinski EA (1999) Extrinsic protein stabilization by the naturally occurring osmolytes b-hydroxyectoine and betaine. Extremophiles 3:191–198
Lippert K, Galinski EA (1992) Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing, and drying. Appl Microbiol Biotechnol 37:61–65
Sauer T, Galinski EA (1998) Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 57:306–313
Vargas Carmen, Argandoña Montserrat, Reina-Bueno Mercedes, Rodríguez-Moya Javier, Fernández-Aunión Cristina, Nieto Joaquin (2008) Unravelling the adaptation responses to osmotic and temperature stress in chromohalobacter salexigens, a bacterium with broad salinity tolerance. Saline Syst 4:14. https://doi.org/10.1186/1746-1448-4-14
Bremer E, Krämer R (2000) Coping with osmotic challenges:osmoregulation through accumulation and release of compatible solutes in bacteria. In: Storz G, Hengge-Aronis R (eds) Bacterial stress responses. ASM Press, Washington, D.C., pp 77–97
Galinski EA (1995) Osmoadaptation in bacteria. Adv Microb Physiol 37:272–328
Roberts MF (2005) Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst 1:5. https://doi.org/10.1186/1746-1448-1-5
Upasani VN, Desai SG (1990) Sambhar Salt Lake: chemical composition of the brines and studies on haloalkaliphlic archaeobacteria. Arch Microbiol 154:589–593
Bouchotroch S, Quesada E, Moral Ad, Llamas I, Bejar V (2001) Halomonasmaura sp. nov., a novel moderately halophilic, exopolysaccharide-producing bacterium. Int J Syst Evolut Microbiol 51:1625–1632
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol 3:208–218
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evolut 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Fenice M, Barghini P, Selbmann L, Federici F (2012) Combined effects of agitation and aeration on the chitinolytic enzymes production by the Antarctic fungus Lecanicillium muscarium CCFEE 5003. Microb Cell Fact 11:12. https://doi.org/10.1186/1475-2859-11-12
Lai MC, Ciulla R, Roberts MF, Sowers KR, Gunsalus RP (1995) Extraction and detection of compatible intracellular solutes. In: Sowers KR, Schreier HT (eds) Archaea a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Kornberg A, Rao N, Ault-Riché D (1999) Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem 68:89–125
Rao NN, Gómez-García MR, Kornberg A (2009) Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem 78:605–647
Seufferheld MJ, Alvarez HM, Farias ME (2008) Role of polyphosphates in microbial adaptation to extreme environments. Appl Environ Microbiol 74:5867–5874
Arahal DR, Castillo AM, Ludwig W, Schleifer KH, Ventosa A (2002) Proposal of Cobetia marina gen. nov., comb. nov., within the family Halomonadaceae, to include the species Halomonas marina. Syst Appl Microbiol 25:207–211
Haba RR, SanchezPorro C, Marquez MC, Ventosa A (2011) Taxonomy of halophiles. In: Horikoshi K (ed) Extremophiles handbook. Springer, Heidelberg, pp 255–308. https://doi.org/10.1007/978-4-431-53898-1-_13
Wang YN, Cai H, Yu SL, Wang ZY, Liu J, Wu XL (2007) Halomonas gudaonensis sp. nov., isolated from a saline soil contaminated by crude oil. Int J Syst Evol Microbiol 57(5):911–915
Mesbah M, Wiegel J (2012) Life under multiple extreme conditions: diversity and physiology of the halophilic alkalithermophiles. Appl Environ Microbiol 78(12):4074–4082
Vickery BH (1926) Simple nitrogeneious constituents of yeast: I. Choline and Nicotinic acid J Biol Chem 68:585–592
Canovas D, Vargas C, Csonka L, Ventosa A, Nieto JJ (1996) Osmoprotectants in Halomonas elongata: high-affinity betaine transport system and choline-betaine pathway. J Bacteriol 178(24):7221–7226
Burkhardt J, Sewald X, Bauer B, Saum SH, Müller V (2009) Synthesis of glycine betaine from choline in the moderate halophile Halobacillus halophilus: co-regulation of two divergent, polycistronic operons. Environ Microbiol Rep 1(1):38–43
Cummings SP, Gilmour DJ (1995) The effect of NaCl on the growth of a Halomonas species: accumulation and utilization of compatible solutes. Microbiology 1:1413–1418
de Lourdes Morenoa M, Garcíaa MT, Ventosaa A, Iglesias-Guerrab F, Melladoa E (2010) The extremely halophilic bacterium SalicolamarasensisIC10 accumulates the compatible solute betaine. Syst Appl Microbiol 33(2010):308–310
Canovas D, Vargas C, Kneip S, Moron MJ, Ventosa A, Bremer E, Niet JJ (2000) Genes for the synthesis of the osmoprotectant glycine betaine from choline in the moderately halophilic bacterium Halomonas elongata DSM 3043. Microbiol 146:455–463
Imhoff JF, Rodriguez-Valera F (1984) Betaine is the main compatible solute of halophilic eubacteria. J Bacteriol 160:478–479
Sharma P, Agarwal V, Medicherla K, Kachhwaha S, Kothari S (2012) Isolation and characterization of Dunaliellaspecies from Sambhar Lake (India) and its phylogenetic position in the genus Dunaliella using 18S rDNA. Natl Acad Sci Lett. https://doi.org/10.1007/s40009-012-0038-6
Acknowledgements
The authors would like to thank CSIR-Institute of Microbial Technology (MTCC), Chandigarh and Belgium Co-ordinate Collection of Microorganism (BCCM), Gent, Belgium for providing necessary facilities for deposition of culture in general repository. The authors would also like to thank MNIT, Jaipur for Transmission Electron Microscopy facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kushwaha, B., Jadhav, I., Verma, H.N. et al. Betaine accumulation suppresses the de-novo synthesis of ectoine at a low osmotic concentration in Halomonas sp SBS 10, a bacterium with broad salinity tolerance. Mol Biol Rep 46, 4779–4786 (2019). https://doi.org/10.1007/s11033-019-04924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04924-2