Abstract
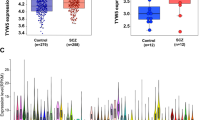
Schizophrenia (SCZ) is a disabling and severe mental illness characterized by abnormal social behavior and disrupted emotions. Similar to other neuropsychological disorders, both genetics and environmental factors interplay so as to develop SCZ. It is acknowledged that genes such as DGKZ are involved in lipid signaling pathways that are the basis of neural activities, memory, and learning and are considered as candidate loci for SCZ. The aim of the present study was to evaluate the expression level and genotypes of DGKZ in patients with SCZ and controls. We used q-PCR to measure the relative expression of DGKZ in blood. To determine DGKZ–rs7951870 genotypes, tetra-ARMS PCR was used. Our results showed a significant difference in DGKZ mRNA ratio between SCZ patients and healthy controls (P = 2 × 10−4). Also, we showed that rs7951870-TT genotype was strongly associated with increased DGKZ expression level (P = 0.038). In conclusion, our findings revealed dysregulation of DGKZ in SCZ patients and a significant correction between the gene expression and DGKZ variant rs7951870.





Similar content being viewed by others
References
Ettinger U et al (2014) Genetics, cognition, and neurobiology of schizotypal personality: a review of the overlap with schizophrenia. Front Psychiatry 5:18
Messias EL, Chen C-Y, Eaton WW (2007) Epidemiology of schizophrenia: review of findings and myths. Psychiatry Clin North Am 30(3):323–338
Cheng C et al (2013) Birth seasonality in schizophrenia: effects of gender and income status. Psychiatry Clin Neurosci 67(6):426–433
Pedersen CB et al (2014) The importance of father’s age to schizophrenia risk. Mol Psychiatry 19(5):530
Gejman PV, Sanders AR, Duan J (2010) The role of genetics in the etiology of schizophrenia. Psychiatry Clin North Am 33(1):35–66
Ripke S et al (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510):421
Kavanagh D et al (2015) Schizophrenia genetics: emerging themes for a complex disorder. Mol Psychiatry 20(1):72
Kim Y et al (2011) Schizophrenia genetics: where next? Schizophr Bull 37(3):456–463
Ruzzo EK, Geschwind DH (2016) Schizophrenia genetics complements its mechanistic understanding. Nat Neurosci 19(4):523
Mirendil H et al (2015) LPA signaling initiates schizophrenia-like brain and behavioral changes in a mouse model of prenatal brain hemorrhage. Transl Psychiatry 5(4):e541
Vogt J et al (2016) Molecular cause and functional impact of altered synaptic lipid signaling due to a prg-1 gene SNP. EMBO Mol Med 8(1):25–38
Müller CP et al (2015) Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta 1851(8):1052–1065
Wong CT, Wais J, Crawford DA (2015) Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur J Neurosci 42(10):2742–2760
Yung YC et al (2015) Lysophosphatidic acid signaling in the nervous system. Neuron 85(4):669–682
Sakane F, Mizuno S, Komenoi S (2016) Diacylglycerol kinases as emerging potential drug targets for a variety of diseases: an update. Front Cell Dev Biol 4:82
Topham MK, Prescott SM (1999) Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem 274(17):11447–11450
Ishisaka M, Hara H (2014) The roles of diacylglycerol kinases in the central nervous system: review of genetic studies in mice. J Pharmacol Sci 124(3):336–343
Bergen SE, Petryshen TL (2012) Genome-wide association studies (GWAS) of schizophrenia: does bigger lead to better results? Curr Opin Psychiatry 25(2):76
Hall MH et al (2014) Neurophysiologic effect of GWAS derived schizophrenia and bipolar risk variants. Am J Med Genet B 165(1):9–18
Ikeda M et al (2011) Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry 69(5):472–478
Potkin SG et al (2008) A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr Bull 35(1):96–108
Goes FS et al (2015) Genome-wide association study of schizophrenia in Ashkenazi Jews. Am J Med Genet B 168(8):649–659
Fromer M et al (2016) Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci 19(11):1442–1453
Kim S et al (2007) Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genom 8(1):413
Sinclair D et al (2012) Glucocorticoid receptor 1B and 1C mRNA transcript alterations in schizophrenia and bipolar disorder, and their possible regulation by GR gene variants. PLoS ONE 7(3):e31720
Ye S et al (2001) An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 29(17):e88–e88
Raben DM, Tu-Sekine B (2008) Nuclear diacylglycerol kinases: regulation and roles. Front Biosci 13:590–597
Weidenhofer J et al (2006) Altered gene expression in the amygdala in schizophrenia: up-regulation of genes located in the cytomatrix active zone. Mol Cell Neurosci 31(2):243–250
Zhang J et al (2004) Neural system-enriched gene expression: relationship to biological pathways and neurological diseases. Physiol Genom 18(2):167–183
Hosak L (2013) New findings in the genetics of schizophrenia. World J Psychiatry 3(3):57
Lencz T et al (2007) Runs of homozygosity reveal highly penetrant recessive loci in schizophrenia. Proc Natl Acad Sci 104(50):19942–19947
Rietschel M et al (2012) Association between genetic variation in a region on chromosome 11 and schizophrenia in large samples from Europe. Mol Psychiatry 17(9):906–917
Schmidt-Kastner R et al (2006) Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res 84(2):253–271
Ip HF et al (2018) Characterizing the relation between expression QTLs and complex traits: exploring the role of tissue specificity. Behav Genet 48(5):374–385
Kim Y et al (2014) A meta-analysis of gene expression quantitative trait loci in brain. Transl Psychiatry 4(10):e459
Johari A et al (2019) The rs1986112 Variant is Associated with Increased RAB8B Gene Expression in Schizophrenic Patients. Clin Lab. https://doi.org/10.7754/Clin.Lab.2018.180832
Acknowledgement
The present work was partially supported by Shahid Beheshti University of Medical Sciences (Tehran, Iran) and Semnan University of Medical Sciences (Semnan, Iran).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alinaghi, S., Alehabib, E., Johari, A.H. et al. Expression analysis and genotyping of DGKZ: a GWAS-derived risk gene for schizophrenia. Mol Biol Rep 46, 4105–4111 (2019). https://doi.org/10.1007/s11033-019-04860-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04860-1